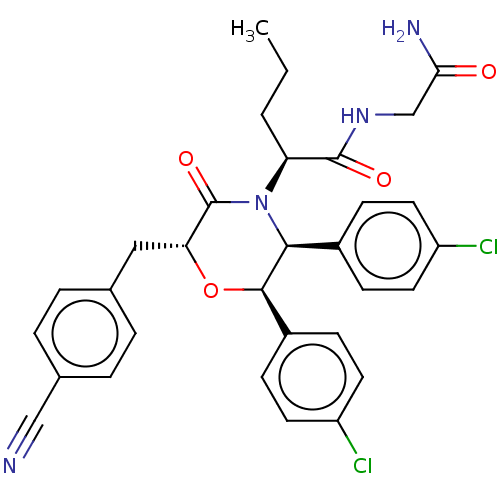
BDBM562788 (R)—N-(2-Amino-2-oxoethyl)-2-((2S,3R,6S)-2,3-bis(4-chlorophenyl)-6-(4-cyanobenzyl)-5-oxomorpholino)pentanamide and (S)—N-(2-amino-2-oxoethyl)-2-((2R,3S,6R)-2,3-bis(4-chlorophenyl)-6-(4-cyanobenzyl)-5-oxomorpholino)pentanamide::US11407721, Example 34
SMILES CCC[C@H](N1[C@H]([C@H](O[C@H](Cc2ccc(cc2)C#N)C1=O)c1ccc(Cl)cc1)c1ccc(Cl)cc1)C(=O)NCC(N)=O
InChI Key InChIKey=UDWYSSZKWZKYFF-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 562788
Found 2 hits for monomerid = 562788
Affinity DataIC50: 486nMAssay Description:This assay was run using the same conditions as the HTRF1 assay except that 20 uL of GST-hMDM2 was incubated with 1.0 ul of diluted compound.More data for this Ligand-Target Pair
Affinity DataIC50: 745nMAssay Description:As the potencies of the HDM2 inhibitors increased, an improved HTRF assay (HTRF2 assay) was developed. All assay conditions remained the same as desc...More data for this Ligand-Target Pair