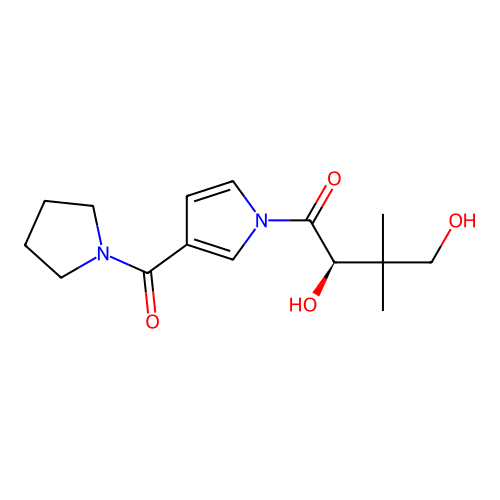
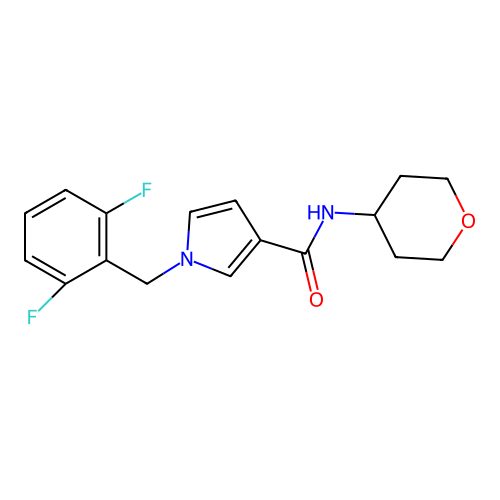
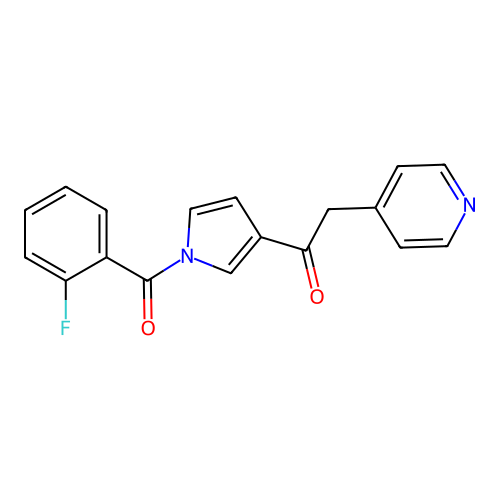
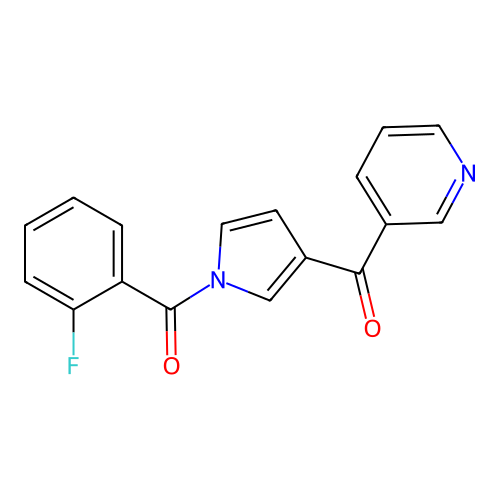
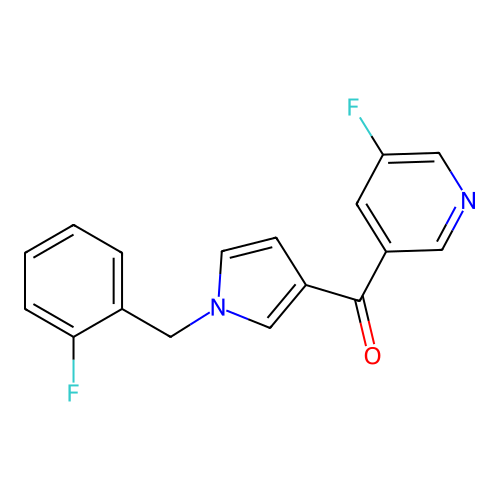
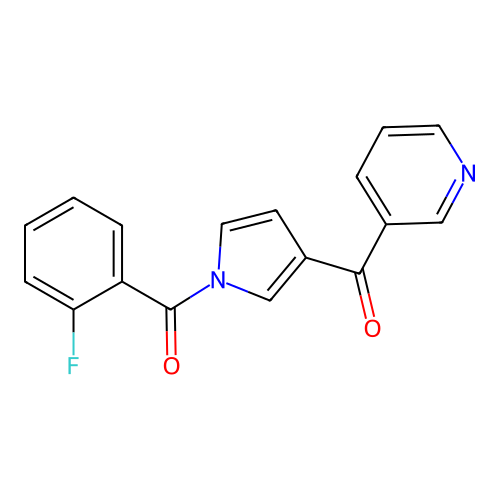
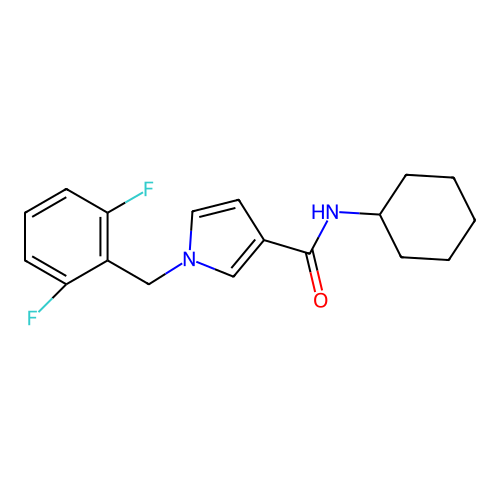
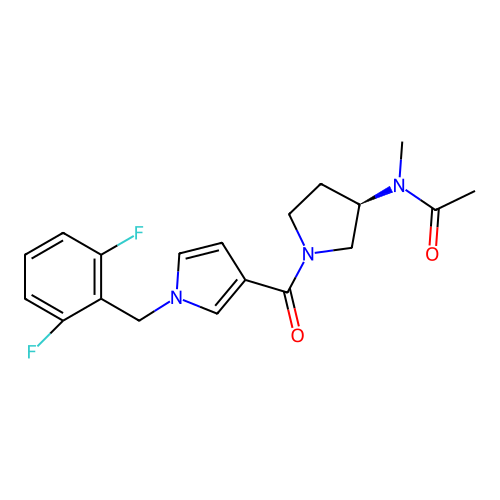
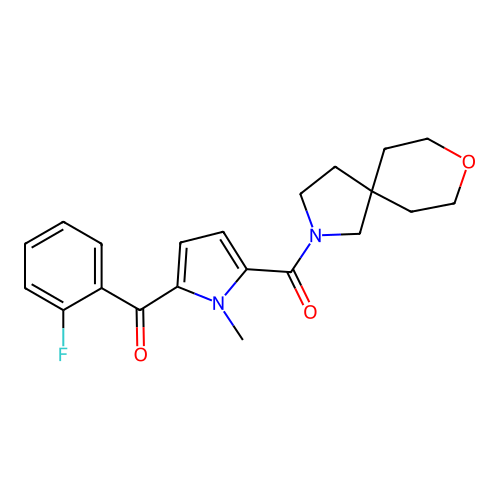
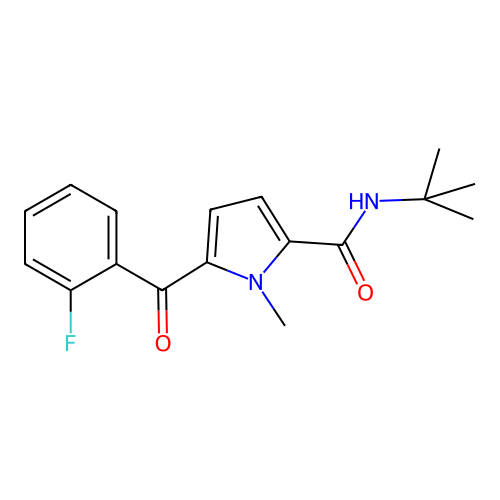
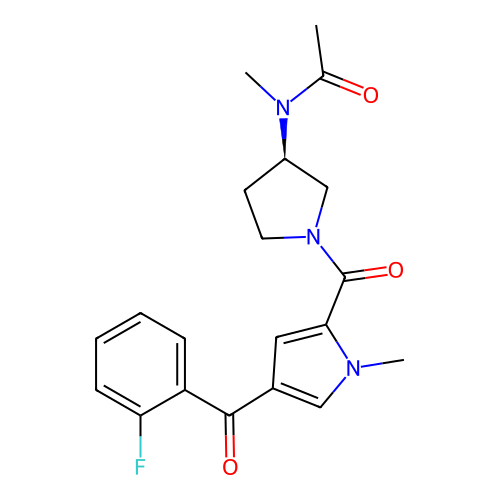
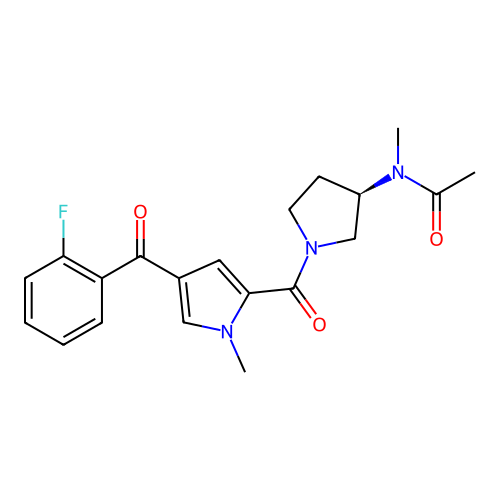
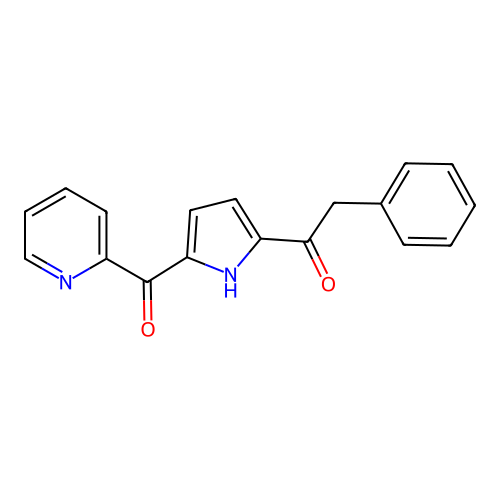
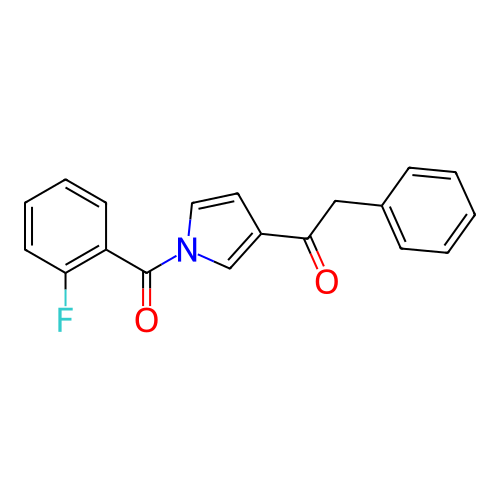
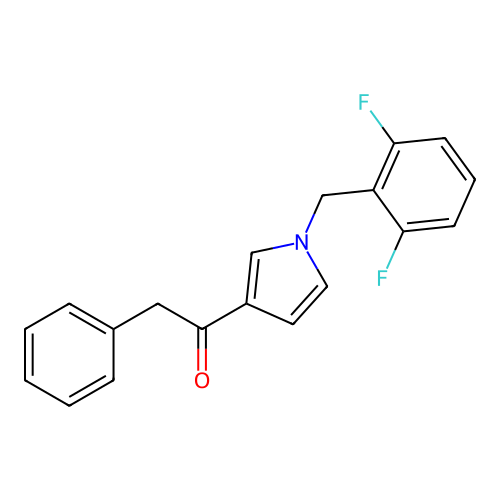
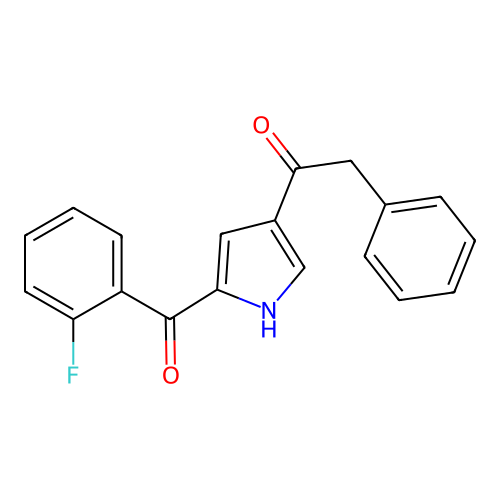
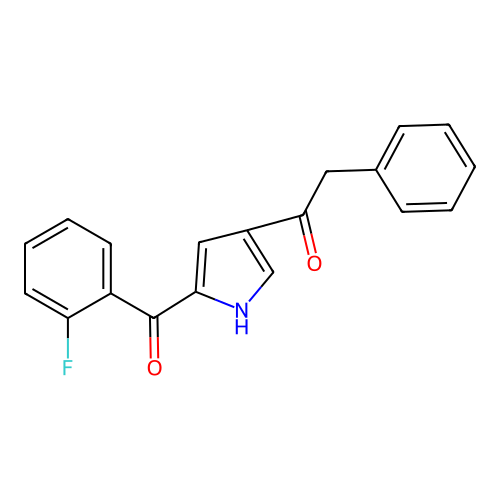
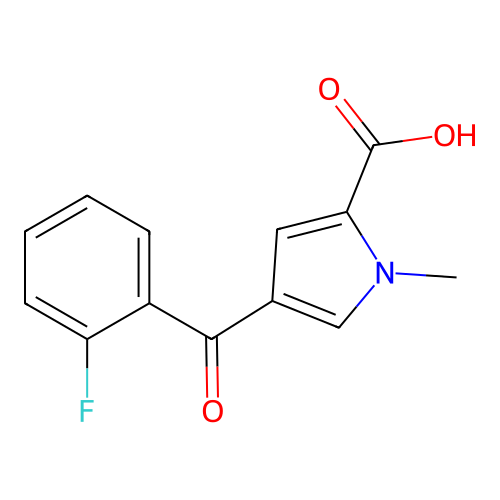
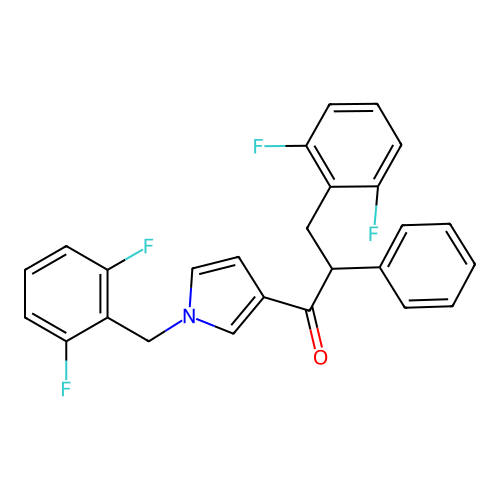
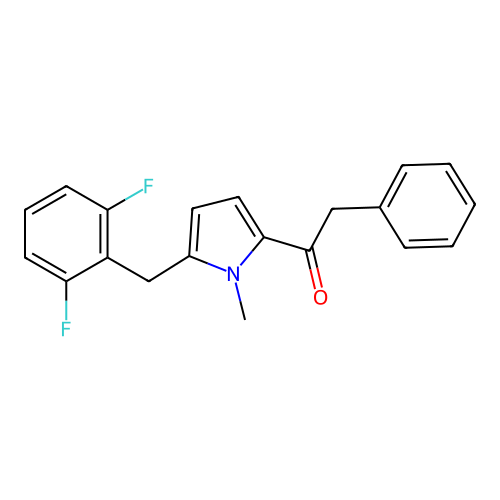Report error Found 102 Enz. Inhib. hit(s) with all data for entry = 12654
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: <100nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: 300nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: 300nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: 300nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: >500nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: >500nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: >500nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataKd: >500nMAssay Description:Table 1 and 5: Test compounds were prepared as 111X stocks in 100% DMSO. Kd values were determined using an 11-point 3-fold compound dilution series ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.53E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 5.06E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 5.52E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 5.83E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 5.92E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 6.03E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 6.10E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 6.26E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 6.34E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 6.55E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 6.72E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 7.13E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 7.19E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 7.37E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 7.53E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 8.09E+4nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Table 3 and 4: The cocktail was incubated in human liver microsome with NADPH regenerating system (NRS) in a shaking bath at 37±2° C. The formation o...More data for this Ligand-Target Pair