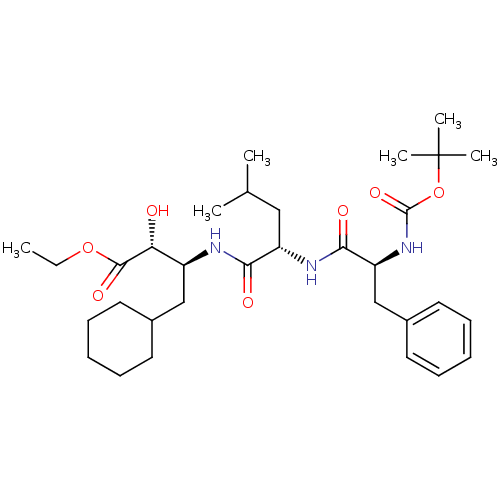
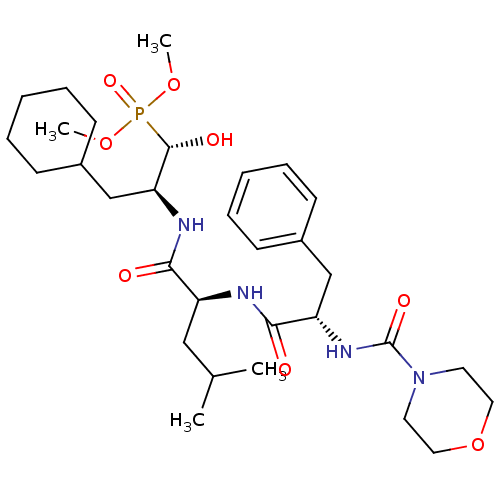
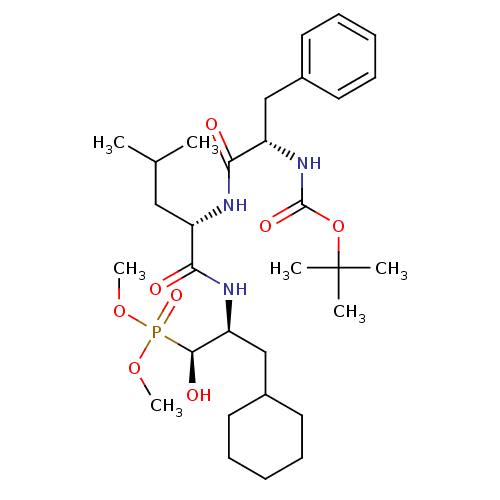
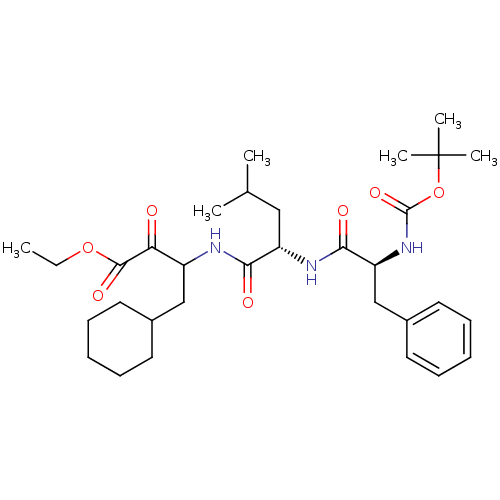
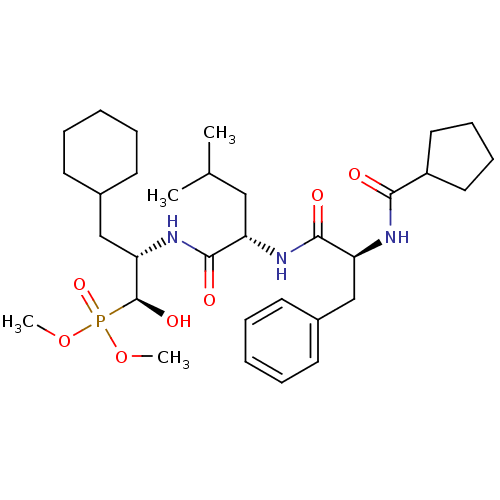
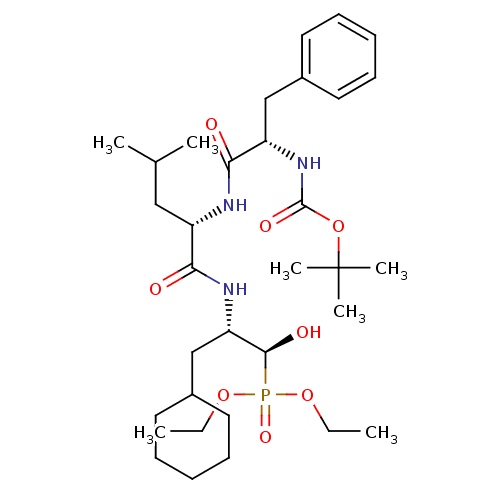
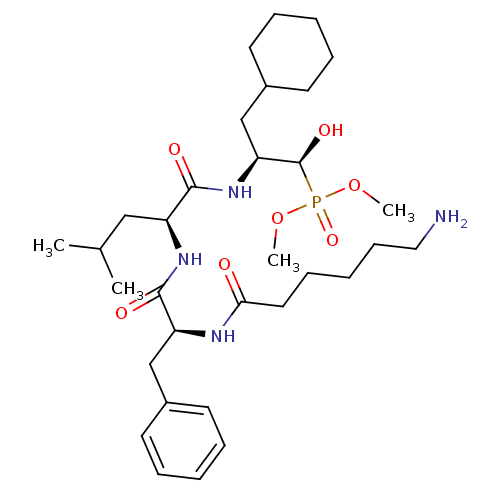
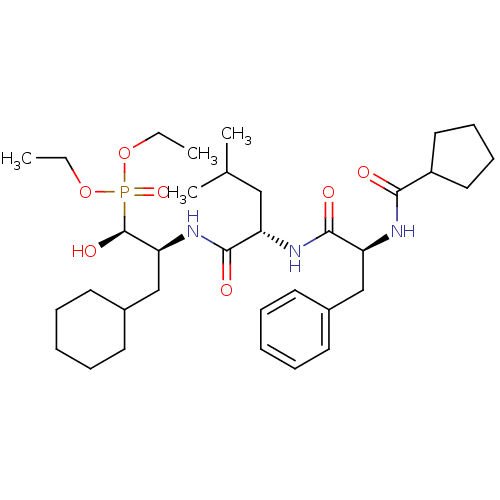
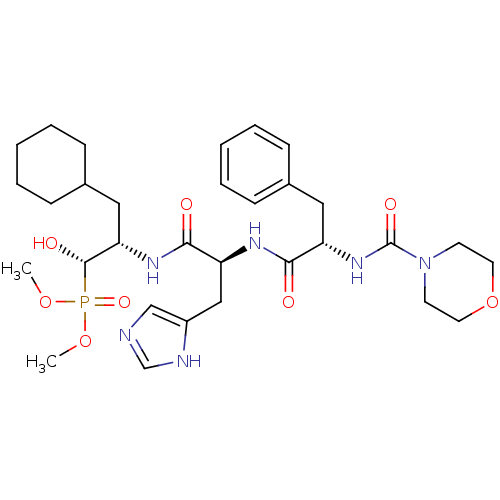
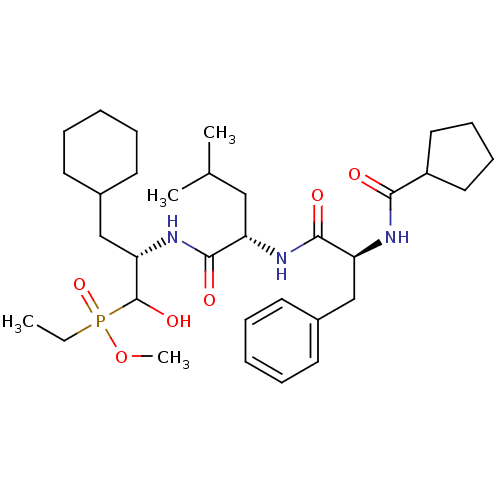
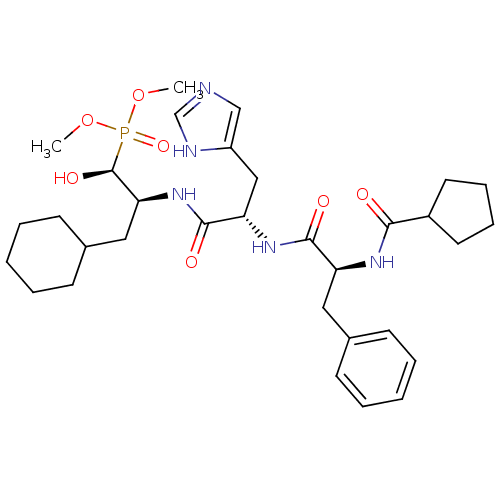
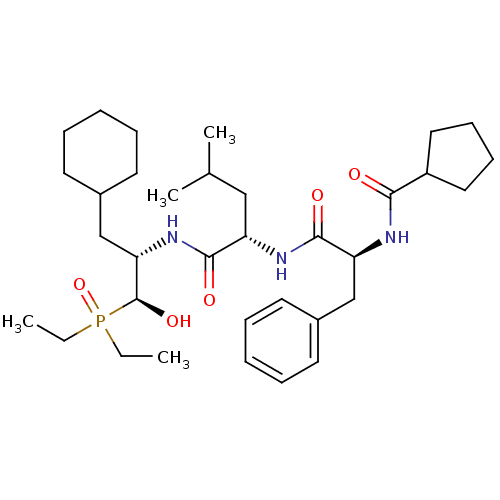
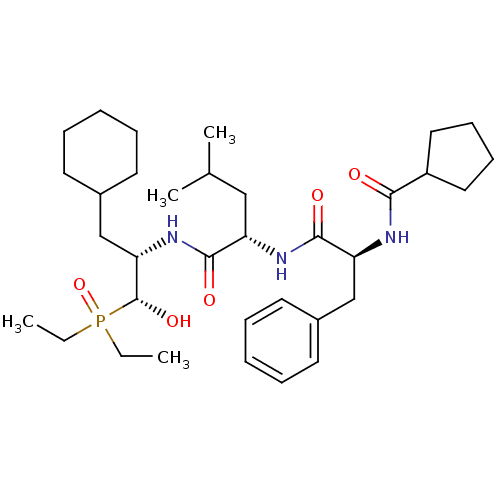
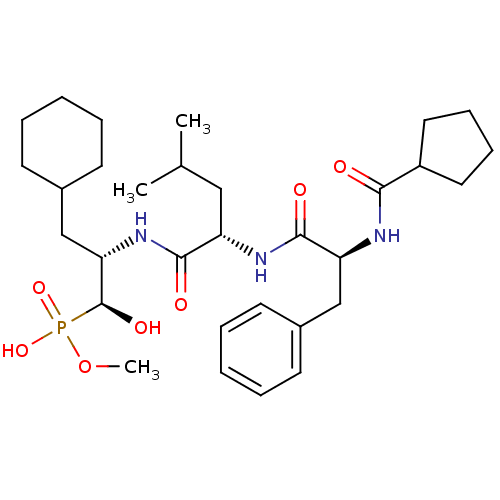
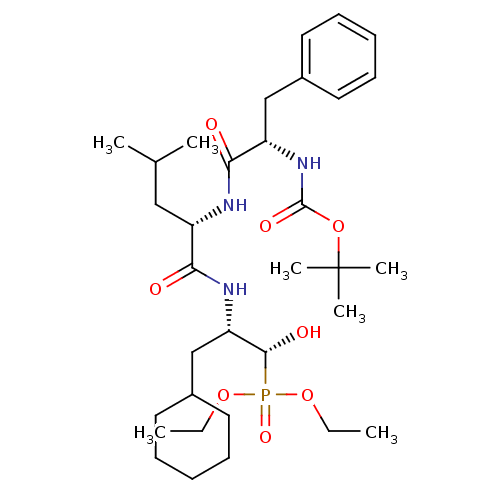
Report error Found 16 Enz. Inhib. hit(s) with all data for entry = 50004887
Affinity DataIC50: 5nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 8.5nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Evaluation of inhibitory activity against human renin.More data for this Ligand-Target Pair
Affinity DataIC50: 15nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 29nMAssay Description:Evaluation of inhibitory activity against human renin.More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Evaluation of inhibitory activity against human renin.More data for this Ligand-Target Pair
Affinity DataIC50: 41nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Evaluation of inhibitory activity against human renin. value in parentheses indicate no. of determinationsMore data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Evaluation of inhibitory activity against human renin. value in parentheses indicate no. of determinationsMore data for this Ligand-Target Pair
Affinity DataIC50: 220nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 400nMAssay Description:Evaluation of inhibitory activity against human renin. value in parentheses indicate no. of determinationsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.20E+3nMAssay Description:Evaluation of inhibitory activity against human renin. value in parentheses indicate no. of determinationsMore data for this Ligand-Target Pair
Affinity DataIC50: 2.70E+4nMAssay Description:Evaluation of inhibitory activity against human reninMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+4nMAssay Description:Evaluation of inhibitory activity against human renin.More data for this Ligand-Target Pair