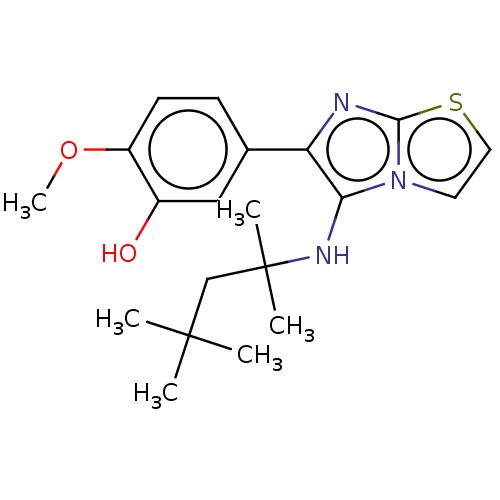
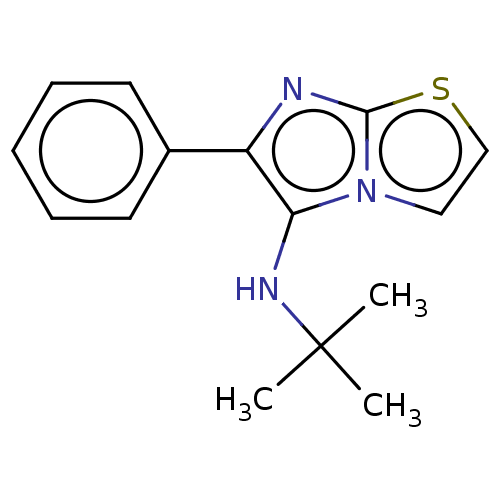
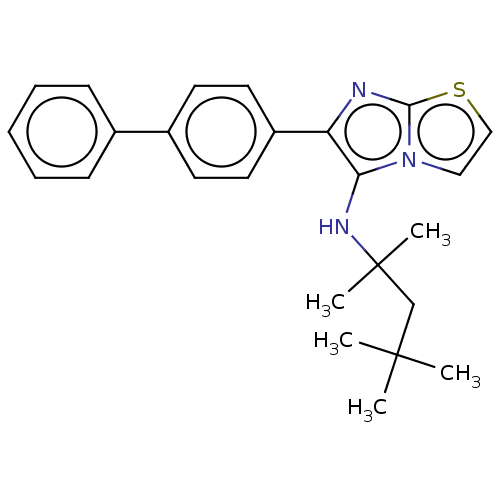
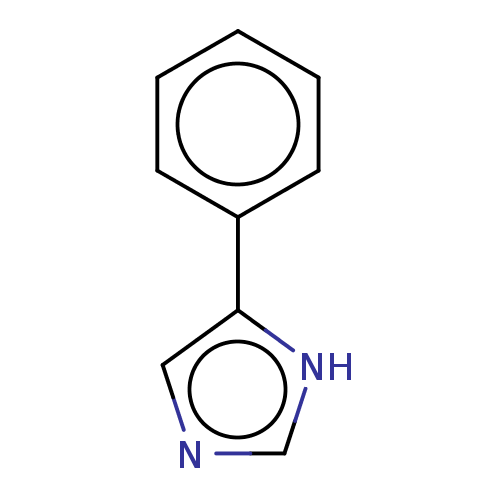
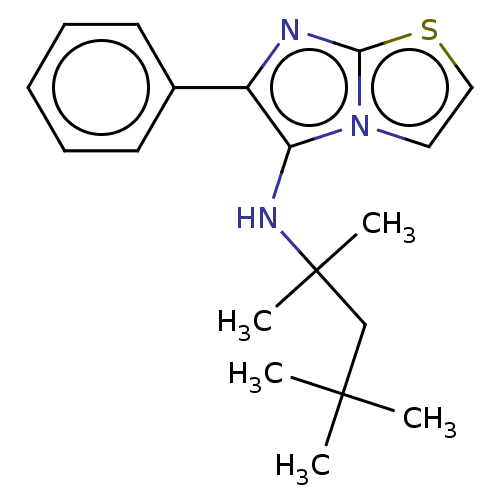
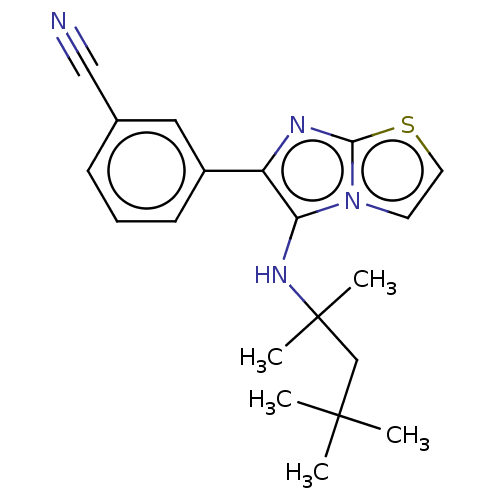
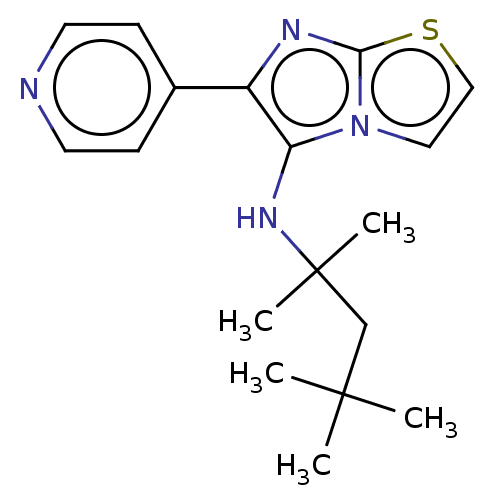
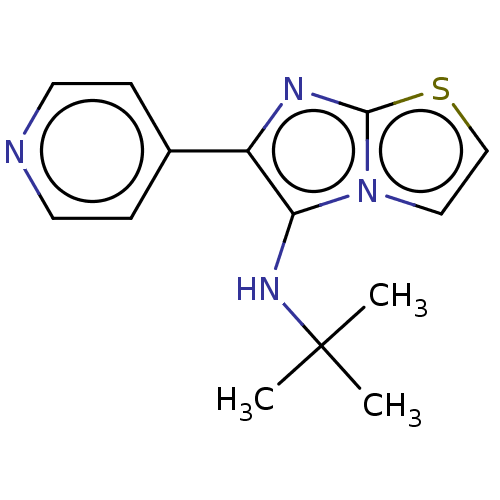
Report error Found 41 Enz. Inhib. hit(s) with all data for entry = 50020849
Affinity DataIC50: 1.70nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.20nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9.60nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 28nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 36nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 56nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 103nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 151nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 240nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 880nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.00E+3nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.30E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.30E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.70E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.80E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 7.30E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.06E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.81E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 2.87E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.65E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 4.77E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 6.91E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 9.08E+5nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.29E+6nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.45E+6nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 1.02E+6nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by UV-visible spectroscopic analysisMore data for this Ligand-Target Pair
Affinity DataKi: 86nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by measuring inhibition constant by U...More data for this Ligand-Target Pair
Affinity DataKi: 160nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by measuring inhibition constant by U...More data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by measuring inhibition constant by U...More data for this Ligand-Target Pair
Affinity DataKi: 2.00E+3nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by measuring inhibition constant by U...More data for this Ligand-Target Pair
Affinity DataKi: 3.70E+4nMAssay Description:Inhibition of human IDO1 using L-tryptophan as substrate assessed as reduction in N-formyl kynurenine formation by measuring inhibition constant by U...More data for this Ligand-Target Pair
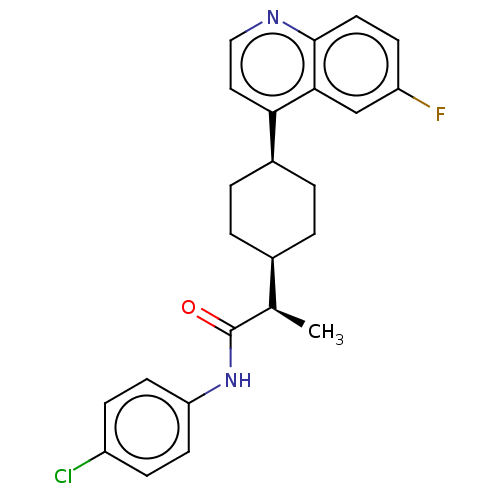
 3D Structure (crystal)
3D Structure (crystal)