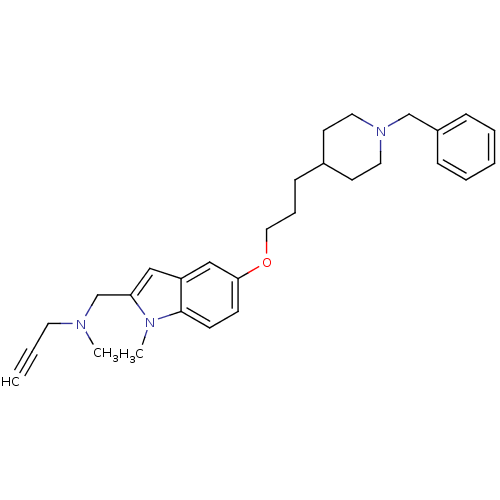
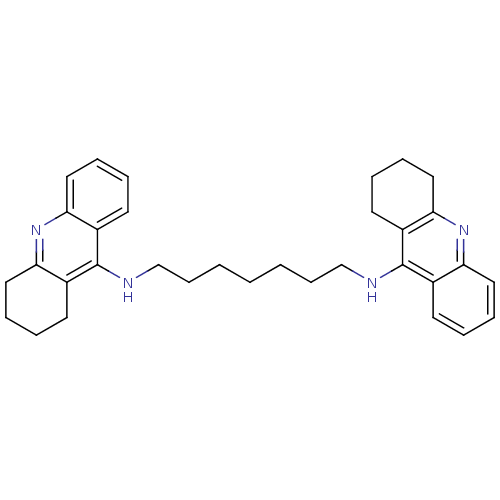
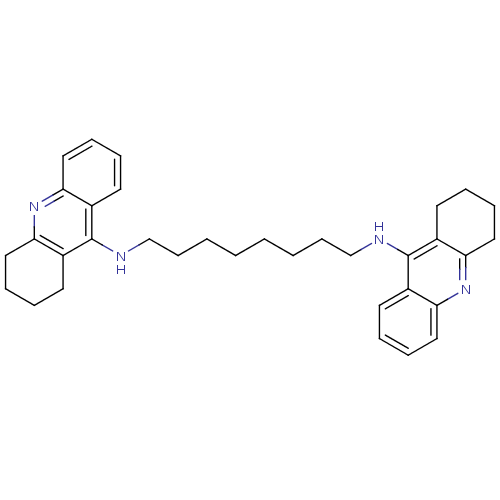
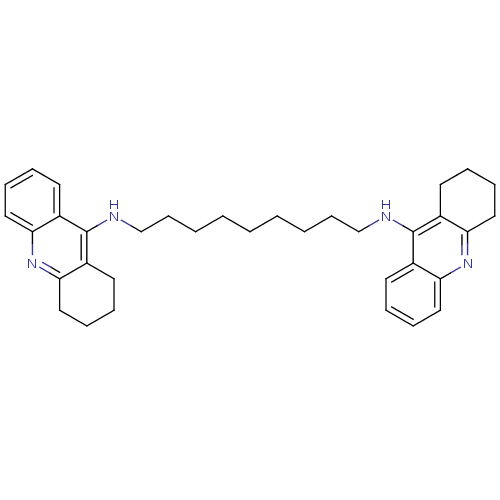
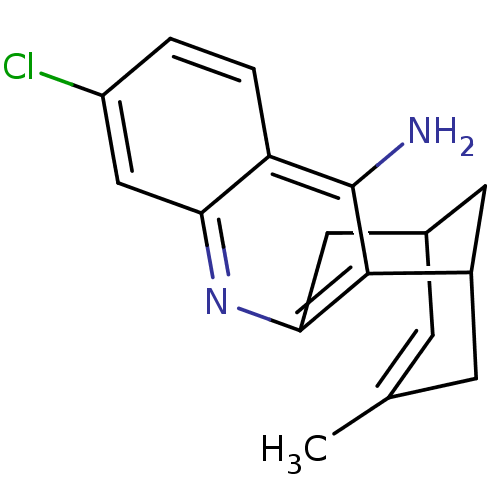
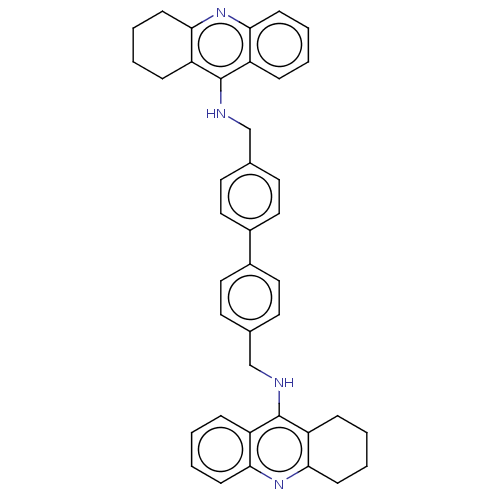
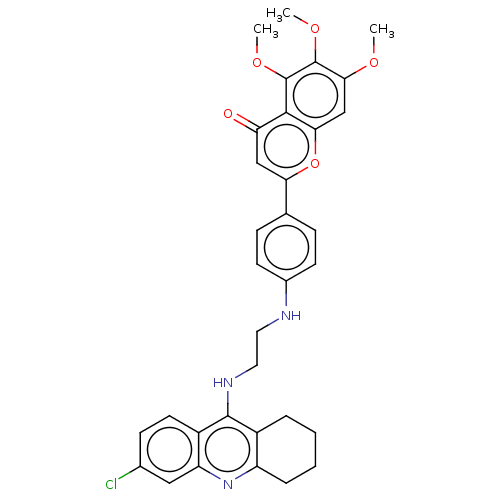
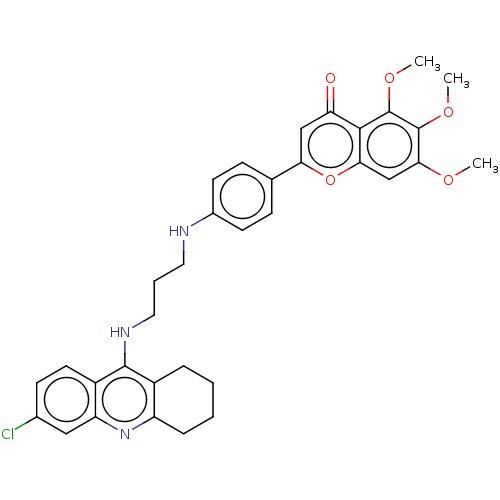
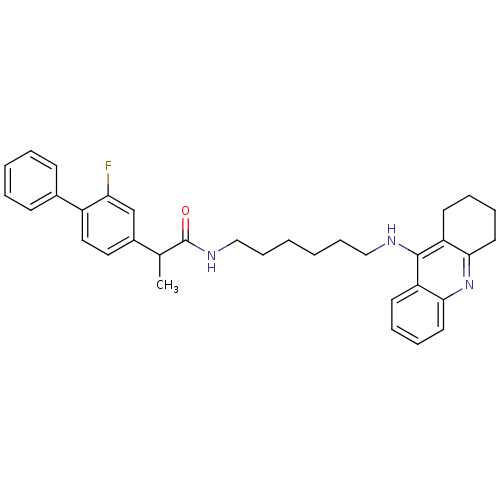
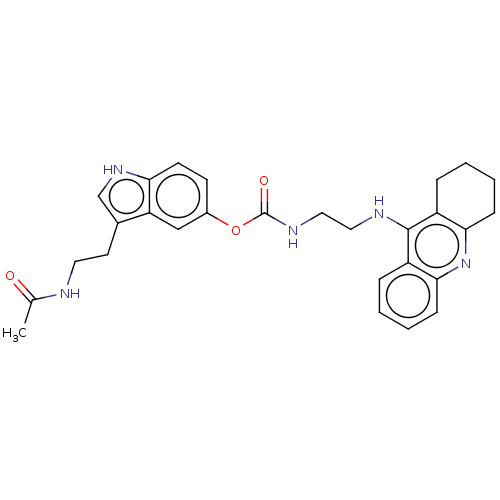
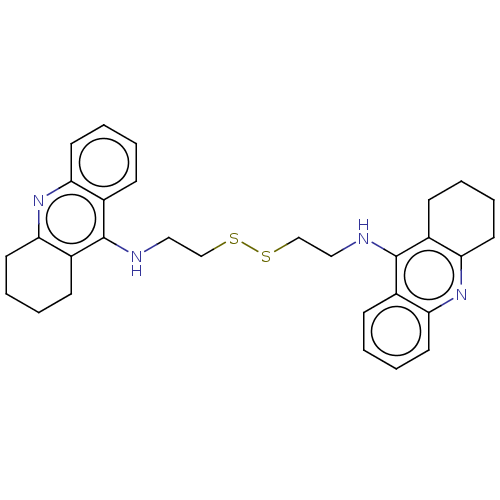
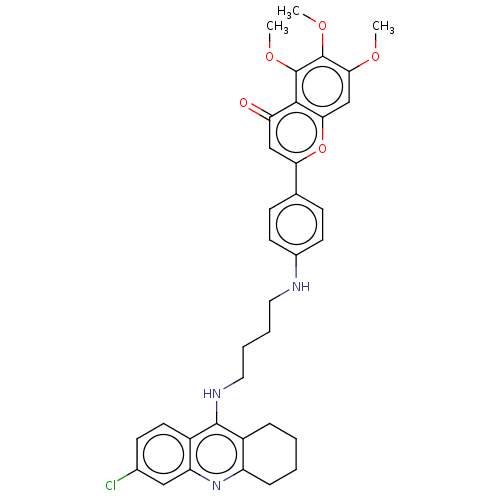
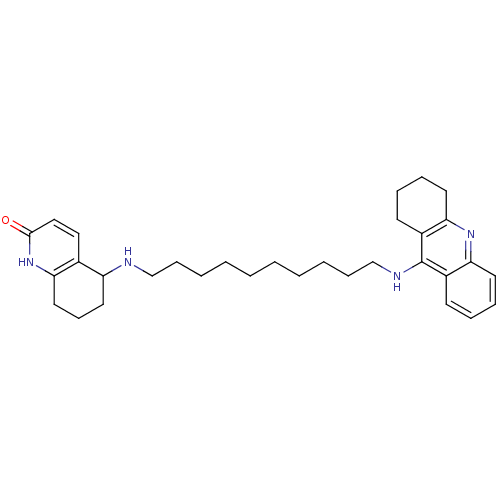
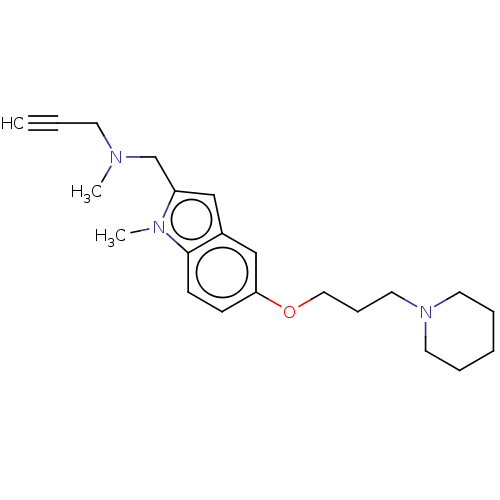
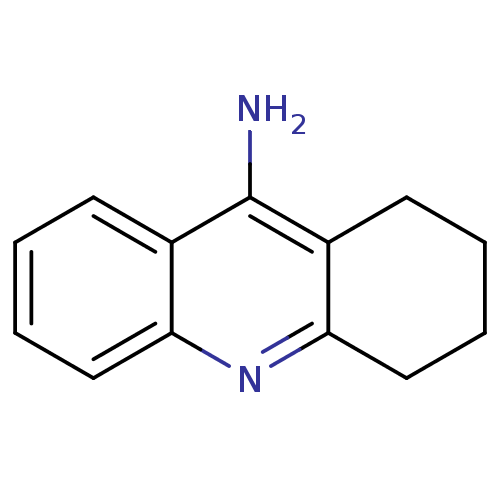
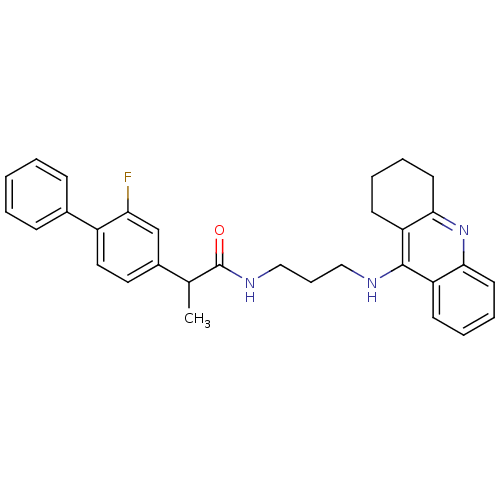
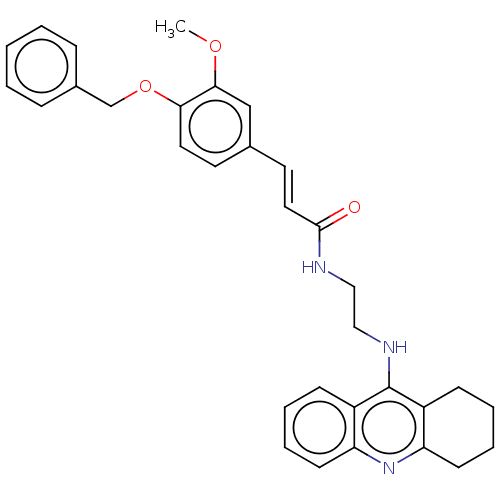
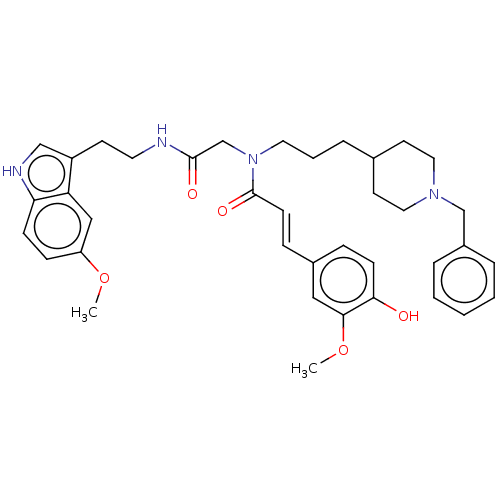
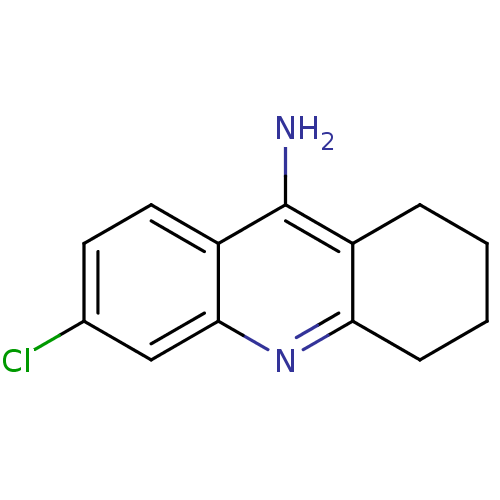
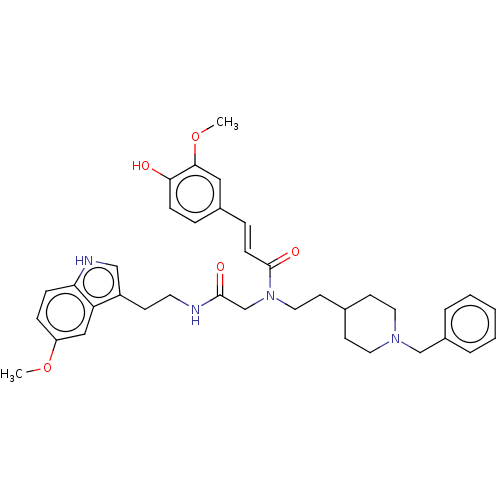
Report error Found 170 Enz. Inhib. hit(s) with all data for entry = 50007595
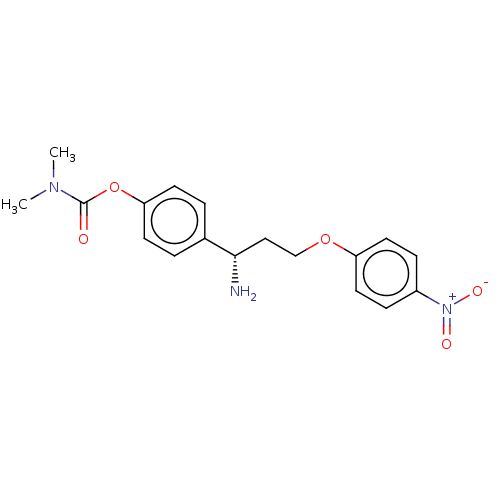
Affinity DataEC50: 0.120nMAssay Description:Agonist activity at human 5H1A receptor expressed in HEK293 cells incubated for 60 mins by Eu-cAMP tracer based LANCE ultra cAMP assayMore data for this Ligand-Target Pair
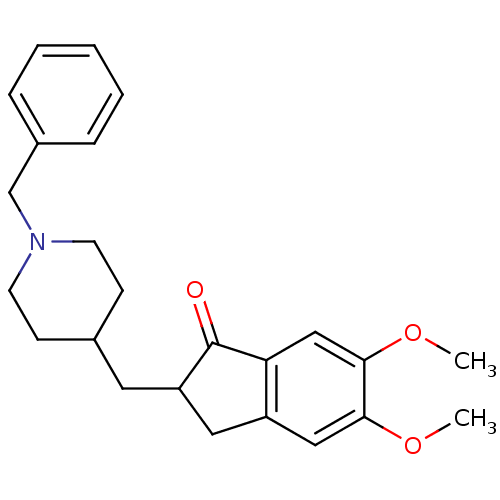
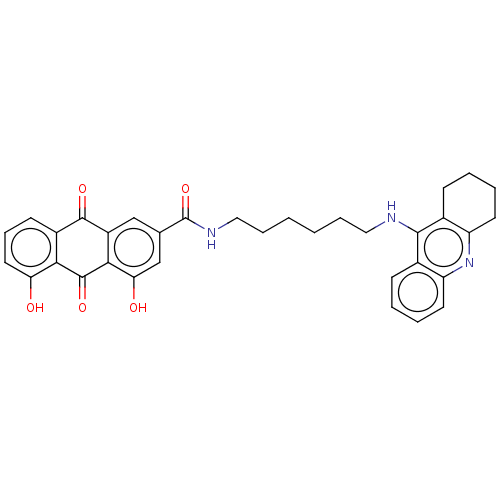
TargetAmine oxidase [flavin-containing] A(Human)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
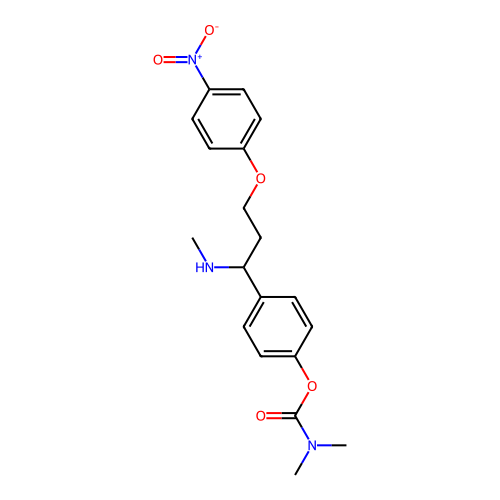
Affinity DataIC50: 0.270nMAssay Description:Inhibition of recombinant human MAOA expressed in baculovirus infected BTI insect cells using p-tyramine as substrate preincubated for 30 mins follow...More data for this Ligand-Target Pair
Affinity DataIC50: 0.400nMAssay Description:Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.660nMAssay Description:Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.770nMAssay Description:Inhibition of rat brain Acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by spectrophotometric m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibition of human Acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.780nMAssay Description:Inhibition of human Acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 0.860nMAssay Description:Inhibition of human serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.60nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E...More data for this Ligand-Target Pair
Affinity DataIC50: 3.60nMAssay Description:Inhibition of human Acetylcholinesterase using acetylthiocholine as substrate preincubated for 20 mins followed by substrate addition by Ellman's met...More data for this Ligand-Target Pair
Affinity DataIC50: 3.70nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated 5 mins followed by substrate addition and measured after 2 m...More data for this Ligand-Target Pair
Affinity DataIC50: 4.20nMAssay Description:Inhibition of bovine erythrocyte acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.23nMAssay Description:Inhibition of bovine erythrocyte acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30nMAssay Description:Inhibition of human plasma butyrylcholinesterase using butyrylthiocholine as substrate preincubated for 30 mins followed by substrate addition and me...More data for this Ligand-Target Pair
Affinity DataIC50: 5nMAssay Description:Inhibition of human acetylcholinesterase using acetylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition by Ellma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.20nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human serum butyrylcholinesterase using butyrylthiocholine iodide as substrate preincubated for 20 mins followed by substrate addition ...More data for this Ligand-Target Pair
Affinity DataIC50: 5.70nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 6nMAssay Description:Inhibition of rat cortex homogenate acetylcholinesterase by Ellman's methodMore data for this Ligand-Target Pair
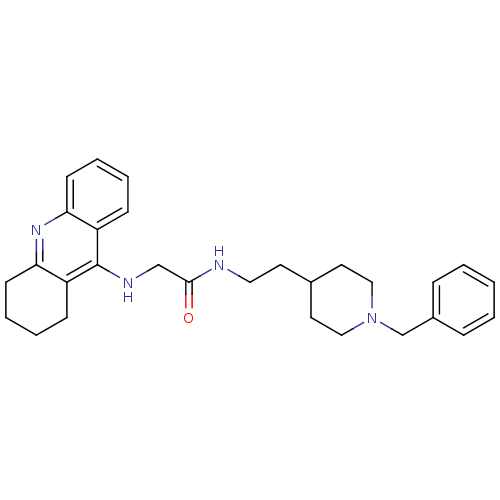
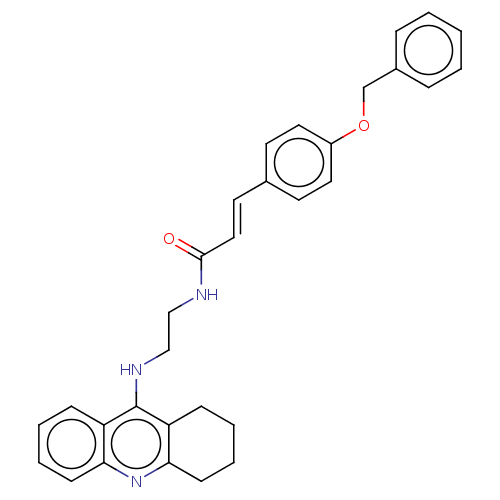
TargetSodium-dependent serotonin transporter(Rat)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of rat synaptosome SERT assessed as reduction in [3H]5-HT uptakeMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rat)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 6nMAssay Description:Inhibition of rat synaptosome SERT assessed as reduction in [3H]5-HT uptakeMore data for this Ligand-Target Pair
Affinity DataIC50: 8.80nMAssay Description:Inhibition of rat cortex homogenate acetylcholinesterase using acetylthiocholine as substrate in presence of BChE inhibitor ethopropazine by Ellman's...More data for this Ligand-Target Pair
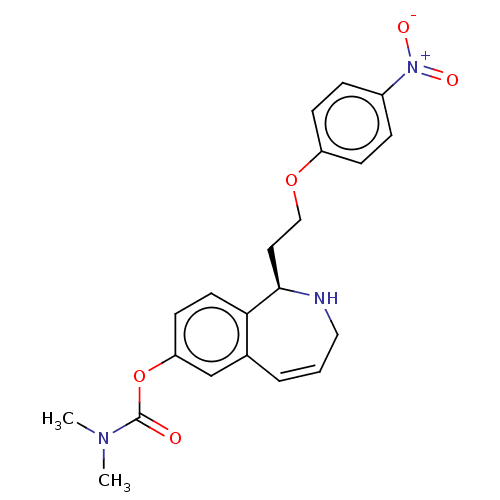
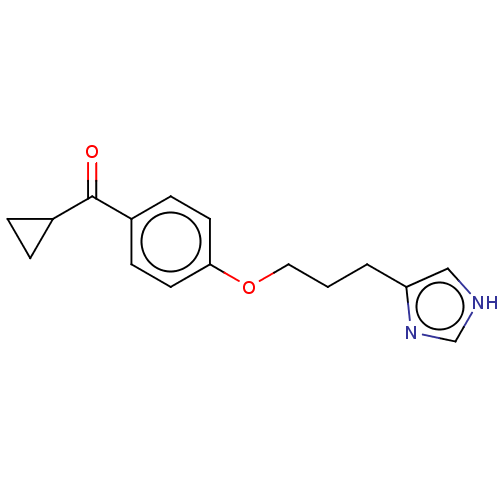
Affinity DataKi: 11nMAssay Description:Displacement of [3H]-N-alpha-methylhistamine from human H3R expressed in HEK293 cell membranes incubated for 90 mins by liquid scintillation counting...More data for this Ligand-Target Pair
Affinity DataIC50: 11nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated followed by substrate addition and measured after 2 mins by ...More data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of human CholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of mouse brain acetylcholinesterase by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of mouse brain acetylcholinesterase by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 14nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated 5 mins followed by substrate addition and measured after 2 m...More data for this Ligand-Target Pair
Affinity DataIC50: 16nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated followed by substrate addition and measured after 2 mins by El...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibition of human butyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 19nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated for 5 mins followed by substrate addition and measured after 2...More data for this Ligand-Target Pair
Affinity DataIC50: 20nMAssay Description:Inhibition of human erythrocyte acetylcholinesterase using acetylthiocholine as substrate preincubated for 5 mins followed by substrate addition by E...More data for this Ligand-Target Pair
Affinity DataIC50: 22nMAssay Description:Inhibition of human butyrylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 23nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 26nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 27nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 6 mins followed by substrate addition and measured up to...More data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
Affinity DataIC50: 30nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate pretreated followed by substrate addition and measured after 2 mins by El...More data for this Ligand-Target Pair
Affinity DataIC50: 31nMAssay Description:Inhibition of human acetylcholinesteraseMore data for this Ligand-Target Pair
TargetAmine oxidase [flavin-containing] A(Human)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 33nMAssay Description:Inhibition of recombinant human MAOA expressed in baculovirus infected BTI insect cells using p-tyramine as substrate by Amplex red reagent/horseradi...More data for this Ligand-Target Pair
Affinity DataIC50: 36.1nMAssay Description:Inhibition of bovine erythrocyte acetylcholinesterase using acetylthiocholine as substrate incubated for 15 mins by Ellman's methodMore data for this Ligand-Target Pair
Affinity DataIC50: 39nMAssay Description:Inhibition of bovine erythrocyte acetylcholinesterase using acetylthiocholine as substrate by Ellman's methodMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rat)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of rat synaptosome SERT assessed as reduction in [3H]5-HT uptakeMore data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rat)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 42nMAssay Description:Inhibition of rat synaptosome SERT assessed as reduction in [3H]5-HT uptakeMore data for this Ligand-Target Pair
Affinity DataIC50: 43nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated followed by substrate addition and measured after 2 mins by ...More data for this Ligand-Target Pair
Affinity DataIC50: 44nMAssay Description:Inhibition of rat serum butyrylcholinesterase using butyrylthiocholine as substrate in presence of AChE inhibitor BW284C51 by spectrophotometric meth...More data for this Ligand-Target Pair
TargetSodium-dependent serotonin transporter(Rat)
Csir-Central Drug Research Institute
Curated by ChEMBL
Csir-Central Drug Research Institute
Curated by ChEMBL
Affinity DataIC50: 44nMAssay Description:Inhibition of rat synaptosome SERT assessed as reduction in [3H]5-HT uptakeMore data for this Ligand-Target Pair
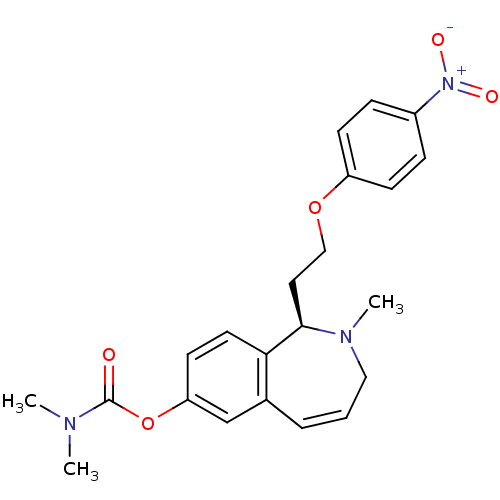
Affinity DataKi: 46nMAssay Description:Displacement of [3H]-N-alpha-methylhistamine from human H3R expressed in HEK293 cell membranes incubated for 90 mins by liquid scintillation counting...More data for this Ligand-Target Pair
Affinity DataIC50: 53nMAssay Description:Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated followed by substrate addition and measured after 2 mins by ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)