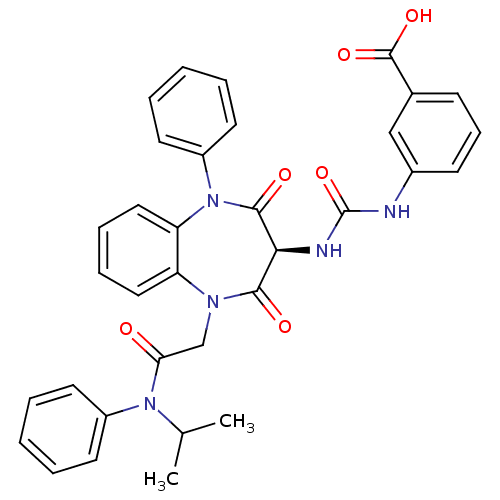
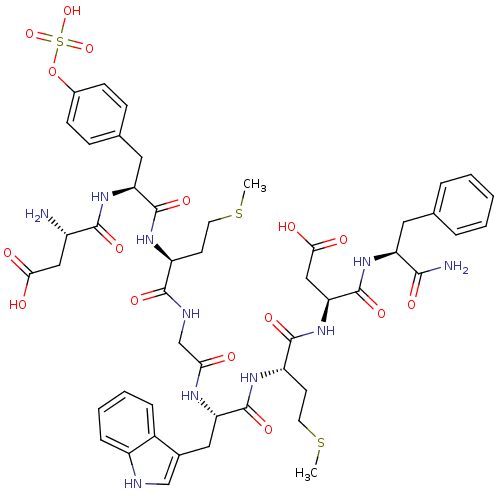
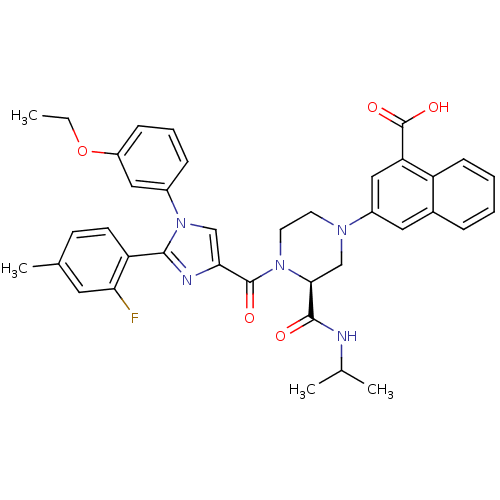
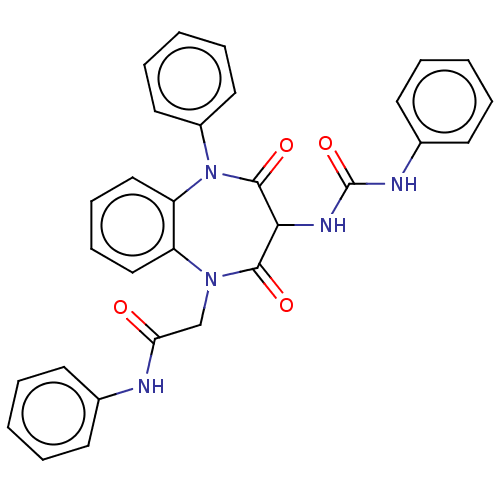
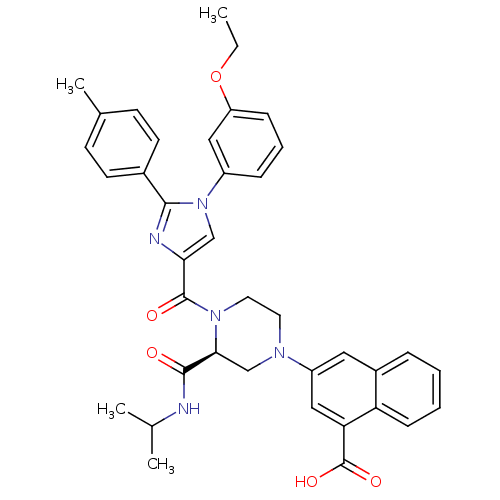
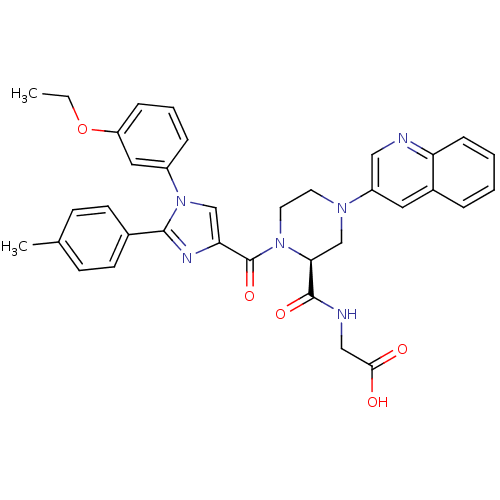
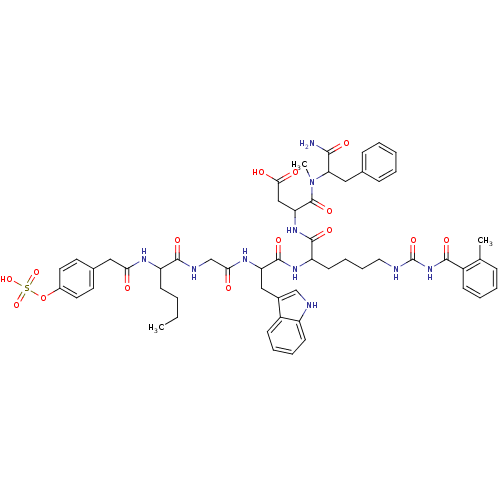
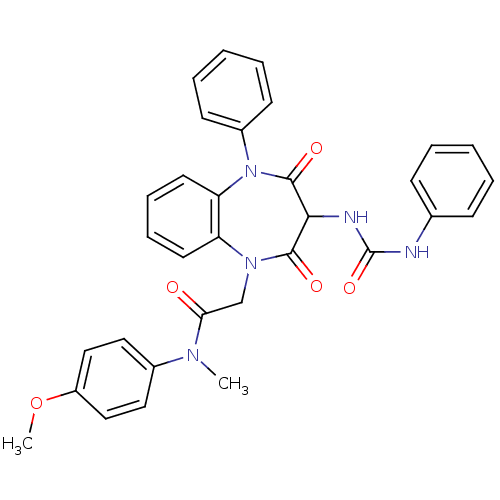
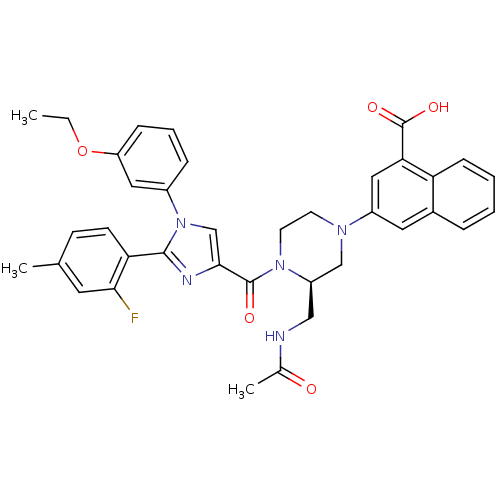
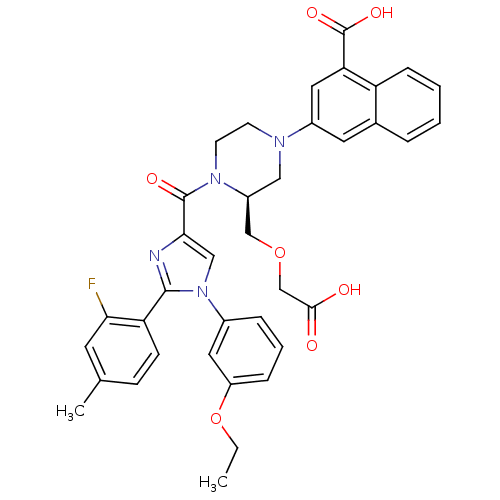
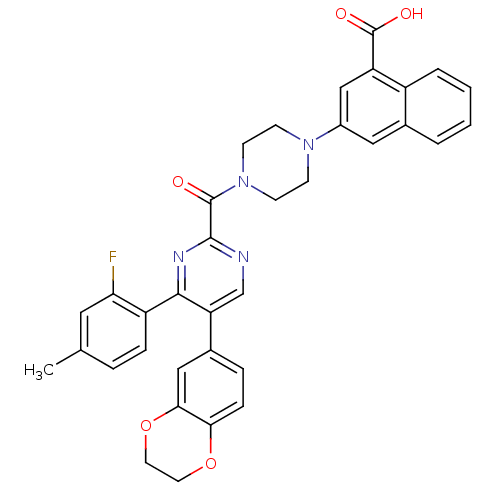
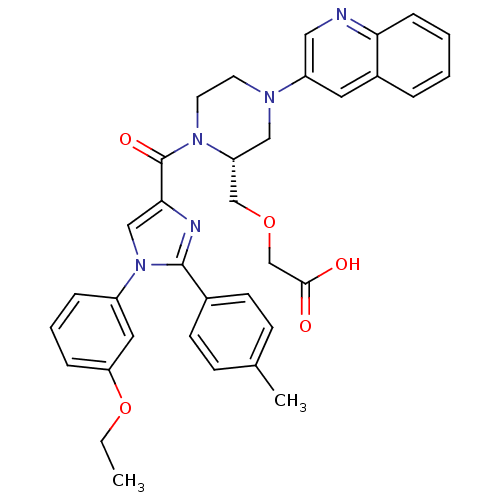
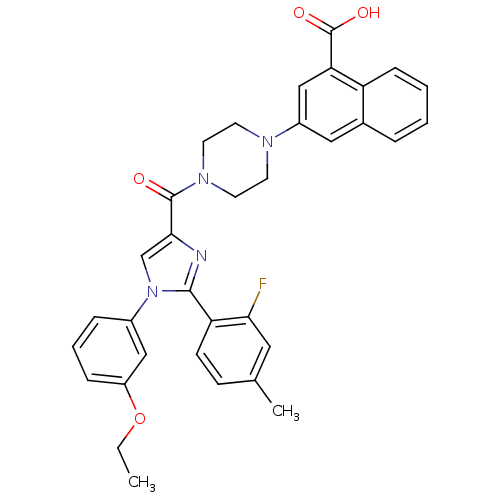
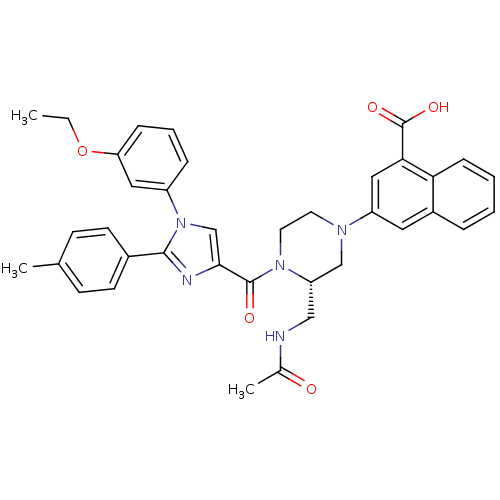
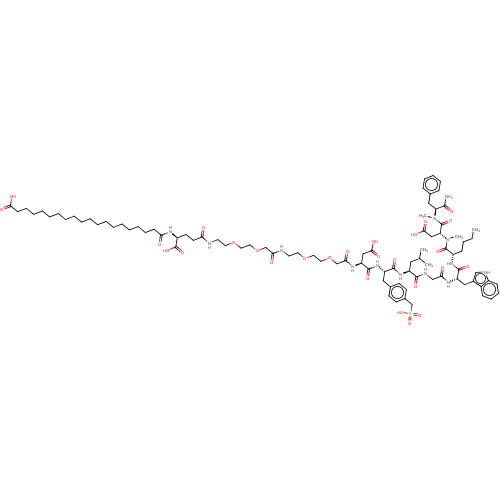
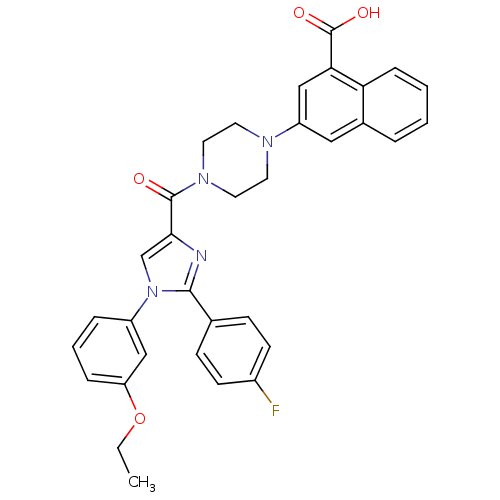
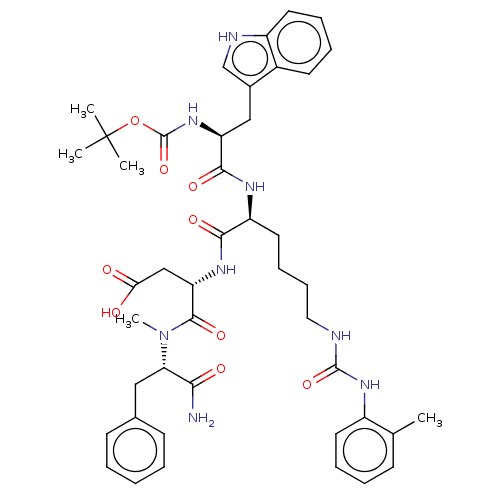
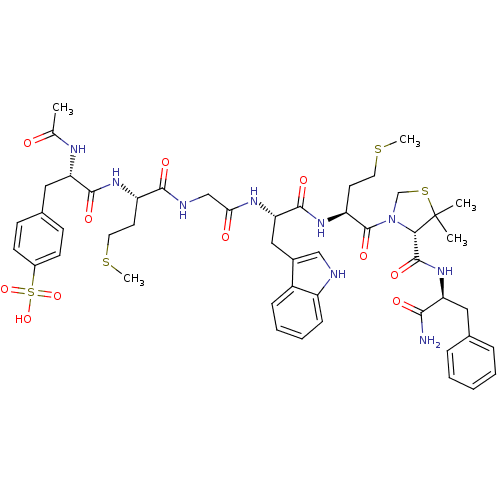
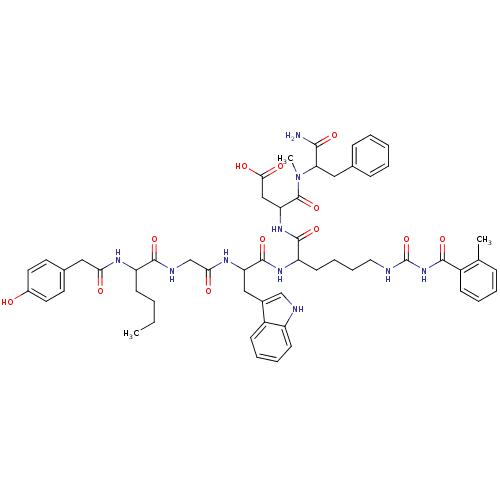
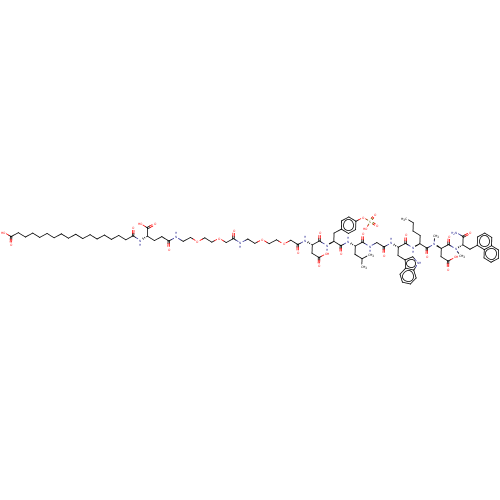Report error Found 3519 Enz. Inhib. hit(s) with Target = 'Cholecystokinin receptor type A'
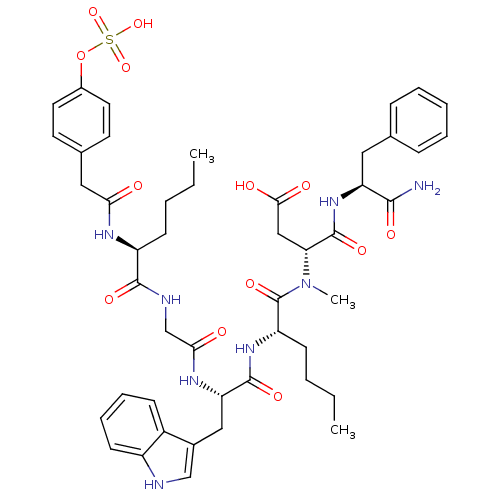
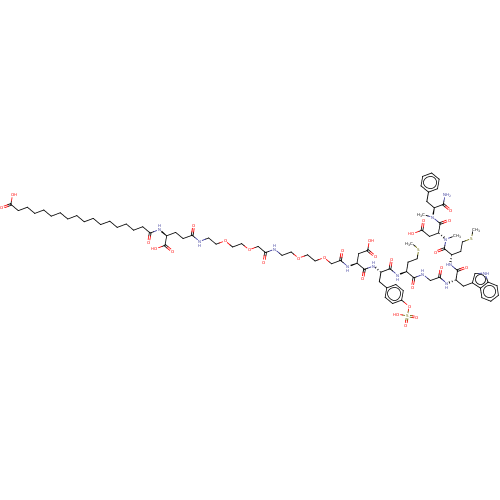
Affinity DataEC50: 0.00200nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.316 nM (Rvb = 7 to 14...More data for this Ligand-Target Pair
TargetCholecystokinin receptor type A/Gastrin/cholecystokinin type B receptor(Human)
Mayo Clinic and Foundation
Curated by ChEMBL
Mayo Clinic and Foundation
Curated by ChEMBL
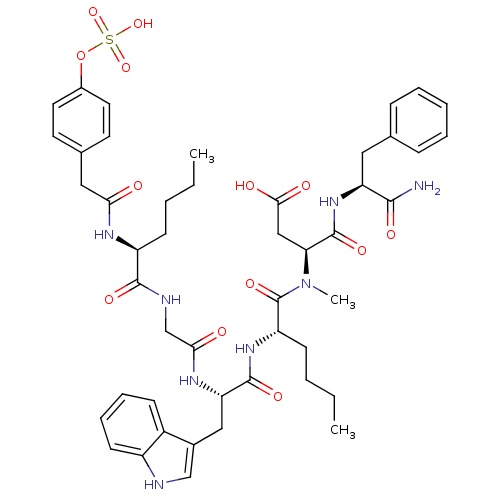
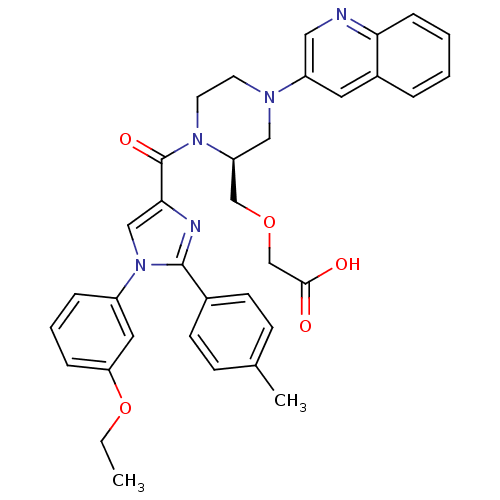
Affinity DataEC50: 0.00900nMAssay Description:Ability to stimulate intracellular calcium response was determined using CCK receptor-bearing chinese hamster ovary cell lineMore data for this Ligand-Target Pair
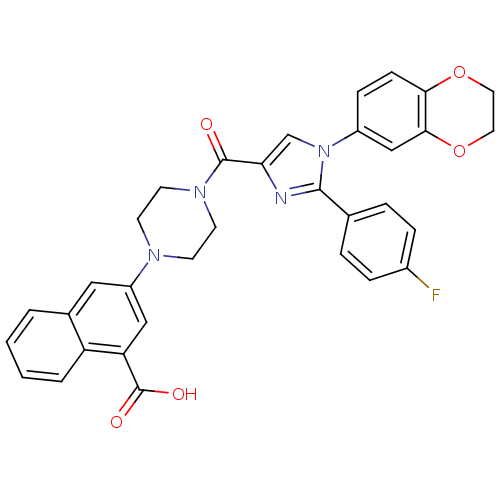
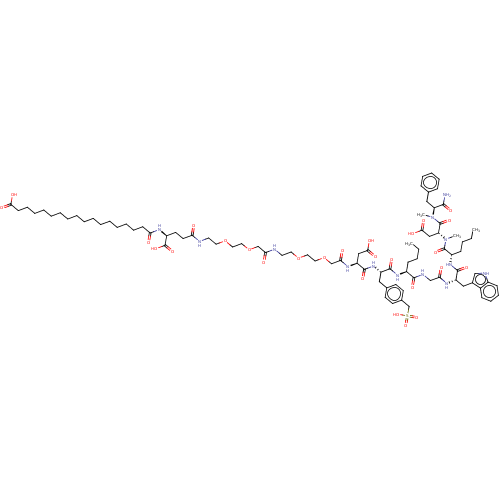
Affinity DataIC50: 0.0100nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
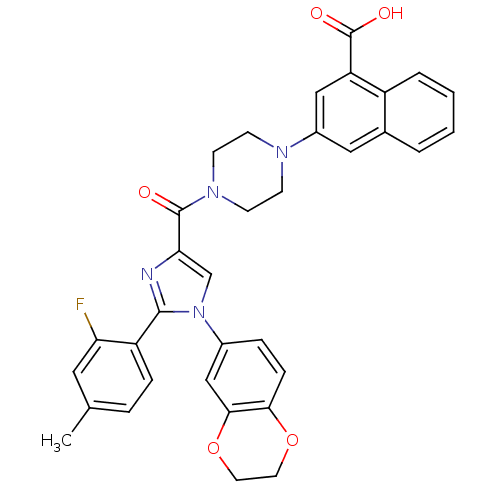
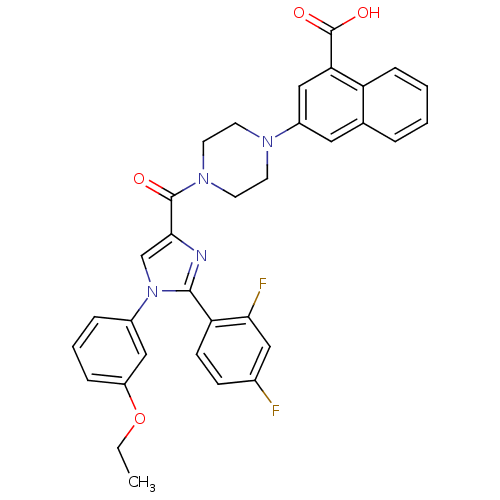
Affinity DataEC50: 0.0100nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.1 nM (Rvb = 7 to 14 p...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0130nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 0.01 nM (Rvb = 7 to 14 ...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0140nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 100 nM (Rvb = 7 to 14 p...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0160nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0180nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0190nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 10 nM (Rvb = 7 to 14 pM...More data for this Ligand-Target Pair
Affinity DataKi: 0.0200nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0200nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 10 nM (Rvb = 7 to 14 pM...More data for this Ligand-Target Pair
Affinity DataEC50: 0.0251nMAssay Description:Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataKi: 0.0300nMAssay Description:Binding affinity for Cholecystokinin type A receptor using [125I]BH-CCK-8 in rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0300nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0330nMAssay Description:Agonist activity at human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0340nMAssay Description:Agonist activity at human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0340nMAssay Description:Displacement of [I125]-CCK8 from human CCK1 receptor expressed in CHO Flip cells after 2 hrs by scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0340nMAssay Description:Agonist activity at mouse CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0390nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 1000 nM (Rvb = 7 to 14 ...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0390nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0400nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0400nMAssay Description:Agonist activity at human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0407nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0410nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0427nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0437nMAssay Description:Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0447nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0480nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:Inhibitory activity against cholecystokinin type A receptor from rat pancreas binding assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0500nMAssay Description:Concentration inhibiting [3H]propionyl-CCK-8 binding to cholecystokinin type A receptor of rat pancreatic membranes.More data for this Ligand-Target Pair
Affinity DataKi: 0.0500nMAssay Description:Inhibition of [125I]BH-CCK-8 binding to Cholecystokinin type A receptor of rat pancreatic tissueMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0501nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0510nMAssay Description:Activity at CCK1R (unknown origin) expressed in CHO cells assessed as CCK EC50 measured as intracellular calcium responses at 100 nM (Rvb = 7 to 14 p...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0520nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0525nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0530nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0530nMAssay Description:Agonist activity at human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0550nMAssay Description:Agonist activity at human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0560nMAssay Description:Agonist activity against human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0580nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0600nMAssay Description:Inhibition of human CCK1 receptorMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0603nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0603nMAssay Description:Agonist activity at human CCK1R expressed in human 1321N1 cells assessed as IP1 accumulation after 1 hr by HTRF assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0630nMAssay Description:Agonist activity at human CCK1 receptor expressed in CHO Flip cells assessed as increase of radio labeled inositol phosphate accumulation by Wallac m...More data for this Ligand-Target Pair
Affinity DataIC50: 0.0631nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 0.0646nMAssay Description:Displacement of [125I]-CCK-8 from human CCK1R expressed in human 1321N1 cell membranes after 2 hrs by SPA assayMore data for this Ligand-Target Pair
Affinity DataEC50: 0.0650nMAssay Description:Agonist activity at human CCK1 receptorMore data for this Ligand-Target Pair