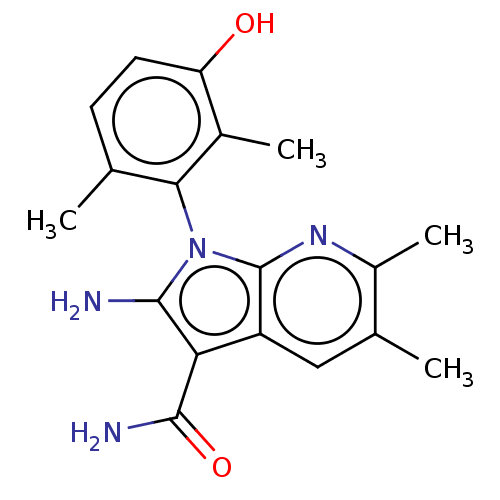
BDBM50598851 CHEMBL5199076::US20240207300, Compound 292
SMILES Cc1ccc(c(c1n2c(c(c3c2nc(c(c3)C)C)C(=O)N)N)C)O
InChI Key InChIKey=ARBRHWRTXPWZGN-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 13 hits for monomerid = 50598851
Found 13 hits for monomerid = 50598851
Affinity DataIC50: 1.5nMAssay Description:Inhibition of human recombinant MYT1 incubated for 60 mins in presence of tracer178 by HTRF analysisMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Repare Therapeutics
Curated by ChEMBL
Repare Therapeutics
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of full length Nano-luc fused PKMYT1 in human HEK-293T cells incubated for 2 hrs by cell based NanoBRET target engagement assayMore data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Repare Therapeutics
Curated by ChEMBL
Repare Therapeutics
Curated by ChEMBL
Affinity DataIC50: 8.40nMAssay Description:Inhibition of recombinant human GST-tagged PKMYT1 (75 to 362 residues) expressed in insect cells using GTDEGIYDVPLLG as substrate preincubated for 15...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Detection of Myt1 kinase activity utilized a recombinant human Myt1 kinase assay measuring the hydrolysis of ATP using a commercially available ADP-G...More data for this Ligand-Target Pair
Affinity DataIC50: 10nMAssay Description:Detection of Myt1 kinase activity utilized a recombinant human Myt1 kinase assay measuring the hydrolysis of ATP using a commercially available ADP-G...More data for this Ligand-Target Pair
TargetMembrane-associated tyrosine- and threonine-specific cdc2-inhibitory kinase(Human)
Repare Therapeutics
Curated by ChEMBL
Repare Therapeutics
Curated by ChEMBL
Affinity DataIC50: 14nMAssay Description:Inhibition of N-terminal recombinant PKMYT1 (76 to 362 residues) in CCNE1 amplified human FU-OV-1 cells assessed as phosphorylation of CDK1 at Thr14 ...More data for this Ligand-Target Pair
Affinity DataIC50: 52nMAssay Description:Inhibition of CDK1 phosphorylation at Thr14 residue in human HCC1569 cells incubated for 6 hrs by Western blot analysisMore data for this Ligand-Target Pair
Affinity DataIC50: 104nMAssay Description:Inhibition of human recombinant Wee1 preincubated with compound for for 15 mins followed by ATP addition and measured after 60 mins by ADP Glo lumine...More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of CREBBP (unknown origin) by TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Repare Therapeutics
Curated by ChEMBL
Repare Therapeutics
Curated by ChEMBL
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of human ERG by patch-clamp assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)