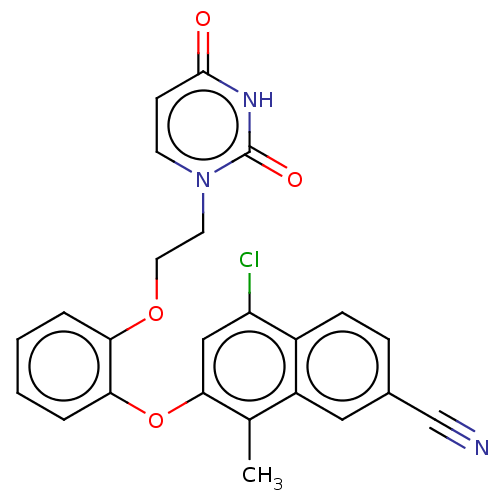
BDBM50501285 CHEMBL3937452
SMILES Cc1c2cc(ccc2c(cc1Oc3ccccc3OCCN4C=CC(=O)NC4=O)Cl)C#N
InChI Key InChIKey=KFUNYNPQHKSQRQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50501285
Found 3 hits for monomerid = 50501285
Affinity DataEC50: 58nMAssay Description:Inhibition of HIV1 reverse transcriptase Y181C mutant infected in human MT2 cells assessed as protection against viral infection by MTT assayMore data for this Ligand-Target Pair
Affinity DataEC50: 280nMAssay Description:Inhibition of HIV1 reverse transcriptase K103N/Y181C double mutant infected in human MT2 cells assessed as protection against viral infection by MTT ...More data for this Ligand-Target Pair
Affinity DataEC50: 280nMAssay Description:Inhibition of HIV1 reverse transcriptase p66/p51 K103N/Y181C mutant infected in human MT2 cells assessed as reduction in viral infection incubated fo...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)