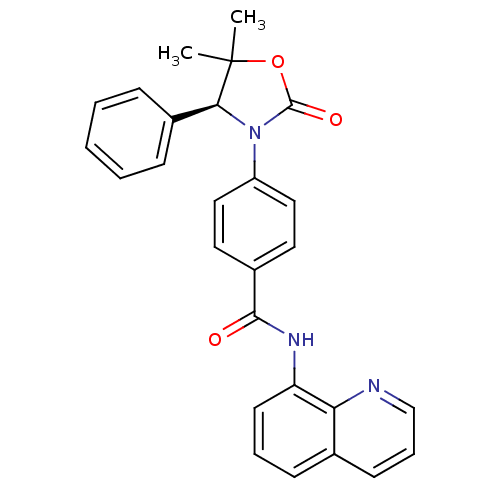
BDBM50434157 CHEMBL2381958::US9340549, 78
SMILES CC1([C@@H](N(C(=O)O1)c2ccc(cc2)C(=O)Nc3cccc4c3nccc4)c5ccccc5)C
InChI Key InChIKey=LUYWAGVRXKSNLL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 50434157
Found 7 hits for monomerid = 50434157
Affinity DataIC50: 189nMT: 2°CAssay Description:The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T...More data for this Ligand-Target Pair
Affinity DataIC50: 198nMT: 2°CAssay Description:The tankyrase 1 biochemical activity of the compounds was assayed in the following assay buffer (50 mM MOPS pH7.5, 100 mM NaCl, 2.5 mM MgCl2, 0.01% T...More data for this Ligand-Target Pair
Affinity DataIC50: 200nMAssay Description:Inhibition of human TNKS1 (1030 to 1317 residues) measured after 20 hrsMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PARP1 (unknown origin) using histone as substrate after 1 hr by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PARP6 (unknown origin) using histone as substrate after 1 hr by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PARP2 (unknown origin) using histone as substrate after 1 hr by luminescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of PARP3 (unknown origin) using histone as substrate after 1 hr by luminescence assayMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)