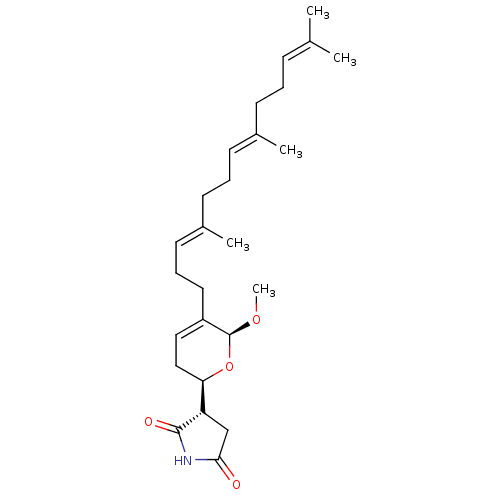
BDBM50345576 CHEMBL1784622::Hippolide B
SMILES [#6]-[#8]-[#6@H]-1-[#8]-[#6@H](-[#6]-[#6]=[#6]-1-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](/[#6])-[#6]-[#6]\[#6]=[#6](\[#6])-[#6])-[#6@@H]-1-[#6]-[#6](=O)-[#7]-[#6]-1=O
InChI Key InChIKey=SHITUGXTMKQHLU-UHFFFAOYSA-N
Data 1 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 1 hit for monomerid = 50345576
Found 1 hit for monomerid = 50345576
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Second Military Medical University
Curated by ChEMBL
Second Military Medical University
Curated by ChEMBL
Affinity DataIC50: 3.97E+4nMAssay Description:Inhibition of recombinant PTP1B catalytic domain using pNPP as substrate assessed as pNP releaseMore data for this Ligand-Target Pair