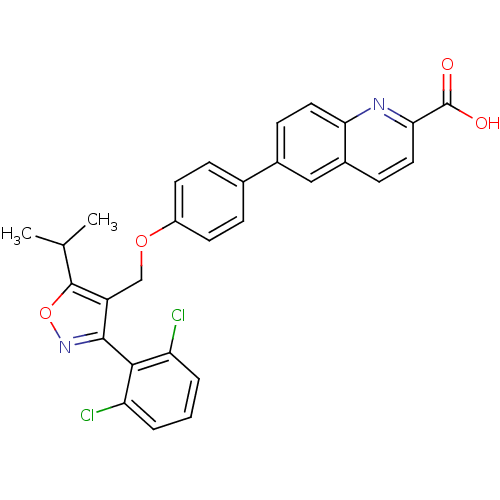
BDBM50336641 6-[4-({[3-(2,6-Dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methyl}oxy)phenyl]-2-quinolinecarboxylic acid::CHEMBL1672448
SMILES CC(C)c1onc(c1COc1ccc(cc1)-c1ccc2nc(ccc2c1)C(O)=O)-c1c(Cl)cccc1Cl
InChI Key InChIKey=SZUHDKKQQZPOGX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 8 hits for monomerid = 50336641
Found 8 hits for monomerid = 50336641
Affinity DataEC50: 50nMAssay Description:Agonist activity at human FXR LBD transfected in african green monkey CV1 cells after overnight incubation by luciferase reporter gene assayMore data for this Ligand-Target Pair
Affinity DataEC50: 50nMAssay Description:Agonist activity at human FXR LBD iexpressed in monkey CV-1 cells assessed as transactivation of luciferase reporter gene expressionMore data for this Ligand-Target Pair
Affinity DataEC50: 120nMAssay Description:Agonist activity at human amino-terminal polyhistidine-tagged FXR alpha LBD (amino acids 237 to 472) assessed as cofactor peptide SRC-1 interaction w...More data for this Ligand-Target Pair
Affinity DataEC50: 120nMAssay Description:Agonist activity at human FXR LBD assessed as SRC1 peptide recruitment by FRET assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.40E+3nMAssay Description:Inhibition of CYP2C9 in human liver microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 2.10E+4nMAssay Description:Inhibition of CYP1A2 in human liver microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2D6 in human liver microsomeMore data for this Ligand-Target Pair
Affinity DataIC50: 3.30E+4nMAssay Description:Inhibition of CYP2C19 in human liver microsomeMore data for this Ligand-Target Pair