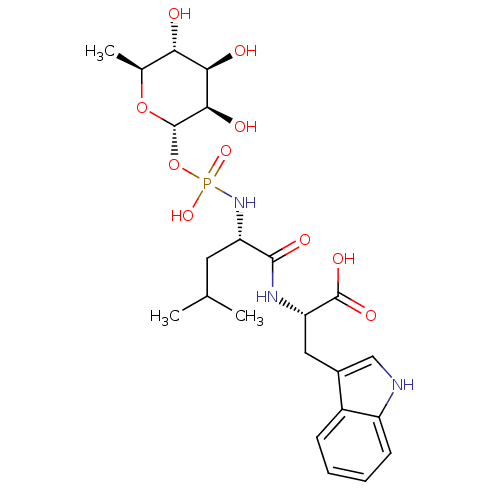
BDBM50251742 (3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-2H-pyran-2-yl hydrogen (S)-1-((S)-1-(1H-indol-3-yl)-3-oxobutan-2-ylamino)-4-methyl-1-oxopentan-2-ylphosphoramidate::(S)-2-{(R)-2-[Hydroxy-((2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid::(S)-2-{(S)-2-[Hydroxy-((2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid::(S)-2-{(S)-2-[Hydroxy-((3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid::(S)-2-{(S)-2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-1-oxo-pentylamino}-3-(1H-indol-3-yl)-propionic acid::(S)-2-{(S)-2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid::(S)-3-(1-Hydroxy-1H-indol-3-yl)-2-{(S)-2-[hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-propionic acid::(phosphoramidon) 2-{2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid::2-{2-[Hydroxy-(3,4,5-trihydroxy-6-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid::2-{2-[Hydroxy-(3,4,6-trihydroxy-5-methyl-tetrahydro-pyran-2-yloxy)-phosphorylamino]-4-methyl-pentanoylamino}-3-(1H-indol-3-yl)-propionic acid( Phosphoramidon)::CHEMBL479579::N-alpha-L-rhamnopyranosyloxy(hydroxyphosphinyl)-L-Leucyl-L-Tryptophan::Phosphoramidon::Phosporamidon::phosphramidon
SMILES CC(C)C[C@H](NP(O)(=O)O[C@@H]1O[C@@H](C)[C@H](O)[C@@H](O)[C@H]1O)C(=O)N[C@@H](Cc1c[nH]c2ccccc12)C(O)=O
InChI Key InChIKey=ZPHBZEQOLSRPAK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 35 hits for monomerid = 50251742
Found 35 hits for monomerid = 50251742
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Novartis Institute For Biomedical Research
Curated by ChEMBL
Helmholtz Institute For Pharmaceutical Research Saarland (HIPS)
Curated by ChEMBL