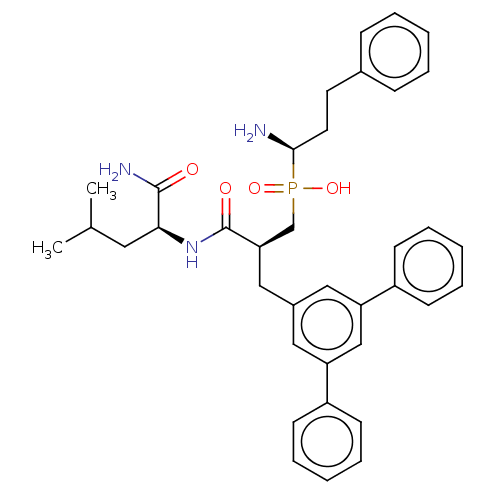
BDBM50245430 CHEMBL4086352
SMILES CC(C)C[C@H](NC(=O)[C@H](Cc1cc(cc(c1)-c1ccccc1)-c1ccccc1)CP(O)(=O)[C@@H](N)CCc1ccccc1)C(N)=O
InChI Key InChIKey=RAXDQSLZABPHNA-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50245430
Found 3 hits for monomerid = 50245430
TargetLeucyl-cystinyl aminopeptidase(Human)
National and Kapodistrian University of Athens
Curated by ChEMBL
National and Kapodistrian University of Athens
Curated by ChEMBL
Affinity DataIC50: 317nMAssay Description:Inhibition of IRAP (unknown origin) expressed in HEK 293S GnTI(-) cells by in vitro fluorimetric assayMore data for this Ligand-Target Pair
TargetEndoplasmic reticulum aminopeptidase 1(Human)
National and Kapodistrian University of Athens
Curated by ChEMBL
National and Kapodistrian University of Athens
Curated by ChEMBL
Affinity DataIC50: 2.12E+4nMAssay Description:Inhibition of ERAP1 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assayMore data for this Ligand-Target Pair
TargetEndoplasmic reticulum aminopeptidase 2(Human)
National and Kapodistrian University of Athens
Curated by ChEMBL
National and Kapodistrian University of Athens
Curated by ChEMBL
Affinity DataIC50: 2.57E+3nMAssay Description:Inhibition of ERAP2 (unknown origin) expressed in Hi5 cells by in vitro fluorimetric assayMore data for this Ligand-Target Pair