BDBM50164482 CHEMBL3800265
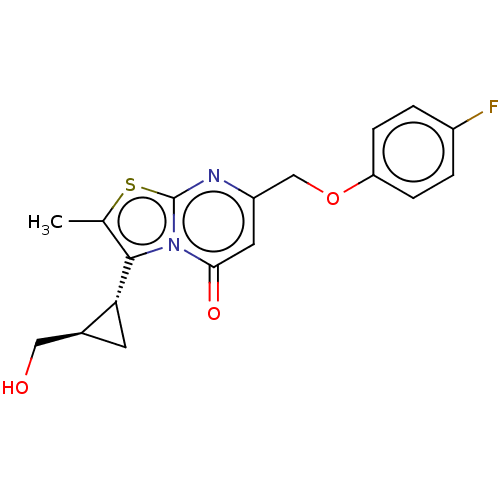
SMILES CC1=C(N2C(=O)C=C(N=C2S1)COc3ccc(cc3)F)[C@@H]4C[C@H]4CO
InChI Key InChIKey=CJDCZVBFZVDWJU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50164482
Found 3 hits for monomerid = 50164482
Affinity DataEC50: 382nMAssay Description:Positive allosteric modulation of GluN1/GluN2A NMDAR (unknown origin) expressed in Dox-inducible cells measured every 5 mins by BD calcium indicator ...More data for this Ligand-Target Pair
Affinity DataEC50: 2.30E+3nMAssay Description:Positive allosteric modulation of human GluA2 AMPAR flop isomer expressed in Dox-inducible cells measured every 5 mins by BD calcium indicator dye ba...More data for this Ligand-Target Pair
Affinity DataEC50: 5.40E+3nMAssay Description:Positive allosteric modulation of human GluA2 AMPAR flip isomer expressed in Dox-inducible cells measured every 5 mins by BD calcium indicator dye ba...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)