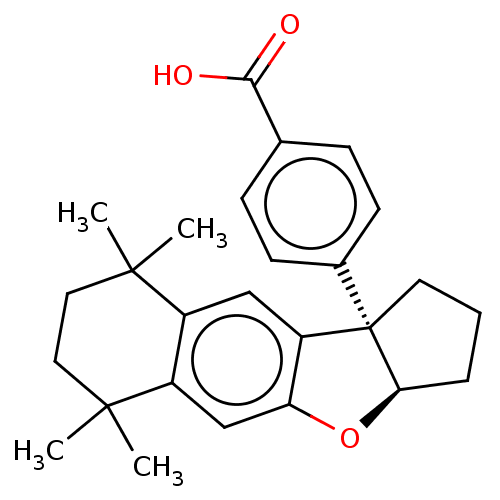
BDBM50157942 CHEMBL3781132
SMILES CC1(CCC(c2c1cc3c(c2)O[C@H]4[C@@]3(CCC4)c5ccc(cc5)C(=O)O)(C)C)C
InChI Key InChIKey=PLLRIXHLFVZTMU-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50157942
Found 2 hits for monomerid = 50157942
Affinity DataEC50: 6.31nMAssay Description:Agonist activity at Renilla luciferase/GFP2-tagged RXRalpha homodimer (unknown origin) expressed in HEK293T cells by BRET2 assayMore data for this Ligand-Target Pair
Affinity DataEC50: 6.30nMAssay Description:Agonist activity at histidine-tagged ligand binding domain of human RXRalpha expressed in Escherichia coli BL21 (DE3) by luciferase reporter gene ass...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)