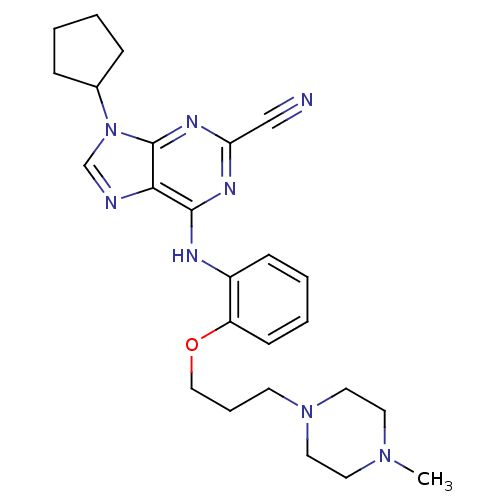
BDBM50156087 9-CYCLOPENTYL-6-{2-[3-(4-METHYL-PIPERAZIN-1-YL)-PROPOXY]-PHENYLAMINO}-9H-PURINE-2-CARBONITRILE::9-cyclopentyl-6-({2-[3-(4-methylpiperazin-1-yl)propoxy]phenyl}amino)-9H-purine-2-carbonitrile::9-cyclopentyl-6-{2-[3-(4-methylpiperazin-1-yl)propoxy]phenylamino}-9H-purine-2-carbonitrile::CHEMBL188139::NVP-ABJ688
SMILES CN1CCN(CC1)CCCOc2ccccc2Nc3c4c(nc(n3)C#N)n(cn4)C5CCCC5
InChI Key InChIKey=VWGLHPDSAYQVRM-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50156087
Found 3 hits for monomerid = 50156087
Affinity DataIC50: 7nMAssay Description:Inhibitory concentration against recombinant human cathepsin K by using Z-Phe-Arg-AMC as synthetic substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 82nMAssay Description:Inhibitory concentration against recombinant human cathepsin L by using Z-Phe-Arg-AMC as synthetic substrateMore data for this Ligand-Target Pair
Affinity DataIC50: 260nMAssay Description:Inhibitory concentration against recombinant human cathepsin S by using Z-Leu-Leu-Arg-AMC as synthetic substrateMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)