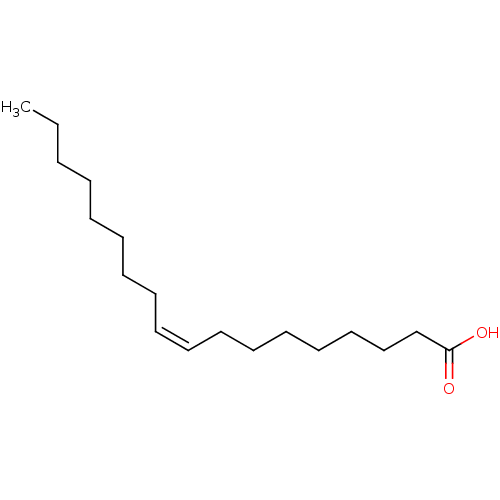
BDBM50150484 (Z)-9-octadecenoic acid::(Z)-Octadec-9-enoic acid::CHEMBL8659::OLEATE::Octadec-9-enoic acid::US20250134842, Compound Oleic acid::oleaic acid::oleicacid
SMILES CCCCCCCC\C=C/CCCCCCCC(=O)O
InChI Key InChIKey=ZQPPMHVWECSIRJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 32 hits for monomerid = 50150484
Found 32 hits for monomerid = 50150484
Affinity DataKd: 200nMAssay Description:Binding affinity to L-FABP high binding affinity site by titration calorimetry methodMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group E member 1/Nuclear receptor-interacting protein 1(Human)
Dartmouth College
US Patent
Dartmouth College
US Patent
Affinity DataEC50: 9.00E+4nMAssay Description:Activation of recombinant His-tagged SIRT6 (unknown origin) deacetylase activity expressed in Escherichia coli using H3K9Ac peptide substrateMore data for this Ligand-Target Pair
Affinity DataEC50: 8.90E+4nMAssay Description:Activation of human SIRT6 deacetylase activity expressed in Escherichia coli BL21(DE3) expressed in Escherichia coli using H3K9Ac peptide substrateMore data for this Ligand-Target Pair
Affinity DataEC50: 3.60E+4nMAssay Description:Agonist activity at human PPARalpha expressed in HEK293 cells by luciferase/beta-galactosidase reporter gene assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 4 group A member 2(Human)
Goethe University Frankfurt
Curated by ChEMBL
Goethe University Frankfurt
Curated by ChEMBL
Affinity DataKd: 5.00E+4nMAssay Description:Binding affinity towards Nurr1 ligand binding domain (unknown origin) measured by tryptophan fluorescence spectroscopyMore data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
University of Copenhagen
Curated by ChEMBL
University of Copenhagen
Curated by ChEMBL
Affinity DataIC50: 6.20E+3nMAssay Description:Inhibition of PTP1B (unknown origin) using pNPP as substrate incubated for 30 mins by photometric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Inhibition of PPAR-alpha (unknown origin) by SPA assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.10E+3nMAssay Description:Displacement of [3H]BRL49653 from Homo sapiens (human) PPARgamma receptor by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Displacement of [3H]GW2433 from Homo sapiens (human) PPARdelta receptor by scintillation proximity assayMore data for this Ligand-Target Pair
Affinity DataIC50: 600nMAssay Description:Displacement of [3H]GW2331 from Homo sapiens (human) PPARalpha receptor by scintillation proximity assayMore data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 2(Rat)
National Research Council (Cnr)
Curated by ChEMBL
National Research Council (Cnr)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Antagonist activity at recombinant rat TRPV2 expressed in HEK293 cells assessed as inhibition of LPC-induced Ca2+ levels preincubated for 5 mins foll...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily V member 2(Rat)
National Research Council (Cnr)
Curated by ChEMBL
National Research Council (Cnr)
Curated by ChEMBL
Affinity DataIC50: 1.00E+4nMAssay Description:Antagonist activity at recombinant rat TRPV2 expressed in HEK293 cells assessed as inhibition of CBD-induced Ca2+ levels preincubated for 5 mins foll...More data for this Ligand-Target Pair
Affinity DataKd: 900nMAssay Description:Binding affinity to L-FABP low binding affinity site by titration calorimetry methodMore data for this Ligand-Target Pair
Affinity DataEC50: >3.50E+5nMAssay Description:Keywords: apoptosis, BH3 domain, Bcl2-A1, BIM, caspase, cancer Primary Collaborator: Todd Golub, Broad Institute, golub@broadinstitute.org Assay Over...More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Rat)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibitory activity against rat liver microsomal Acyl coenzyme A:cholesterol acyltransferaseMore data for this Ligand-Target Pair
Affinity DataIC50: 2.00E+5nMAssay Description:Inhibition of pig pancreatic trypsin after 15 minsMore data for this Ligand-Target Pair
Affinity DataIC50: 8.00E+4nMAssay Description:Inhibition of amidolytic activity of human tissue factor/human factor 7aMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of sheep COX2-mediated prostaglandin biosynthesis using [1-14C]arachidonic acidMore data for this Ligand-Target Pair
Affinity DataIC50: 5.00E+5nMAssay Description:Inhibition of bovine COX1-mediated prostaglandin biosynthesis using [1-14C]arachidonic acidMore data for this Ligand-Target Pair
Affinity DataIC50: 3.10E+4nMAssay Description:Inhibition of DNA topoisomerase 1More data for this Ligand-Target Pair
Affinity DataIC50: 3.27E+4nMAssay Description:Inhibition of aromatase in human placental microsomes by radiometric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.61E+4nMAssay Description:Displacement of 8-anilino-1-naphthalene-sulfonic acid from human His-FABP4 expressed in Escherichia coli BL21 (DE3) cells after 3 minsMore data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Human)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibitory activity against human Acyl coenzyme A:cholesterol acyltransferase 2More data for this Ligand-Target Pair
TargetSterol O-acyltransferase 1(Human)
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibitory activity against human Acyl coenzyme A:cholesterol acyltransferase 1More data for this Ligand-Target Pair
Affinity DataKi: 180nMAssay Description:Displacement of 1-anilinonaphthalene-8-sulphonic acid from rat recombinant L-FABP high binding affinity site expressed in Escherichia coli BL21 by co...More data for this Ligand-Target Pair
Affinity DataKi: 185nMAssay Description:Displacement of 1,8-ANS from aFABP by fluorescence based-assayMore data for this Ligand-Target Pair
TargetFatty acid-binding protein 5(Human)
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Bristol-Myers Squibb Pharmaceutical Research Institute
Curated by ChEMBL
Affinity DataKi: 248nMAssay Description:Displacement of 1,8-ANS from eFABP by fluorescence based-assayMore data for this Ligand-Target Pair
Affinity DataKi: 1.50E+3nMAssay Description:Displacement of 1,8-ANS from His6-tagged FABP4 (unknown origin) expressed in Escherichia coli BL21(DE3) cells by fluorescence assayMore data for this Ligand-Target Pair
Affinity DataKi: 2.90E+3nMAssay Description:Displacement of 1-anilinonaphthalene-8-sulphonic acid from rat recombinant L-FABP low binding affinity site expressed in Escherichia coli BL21 by com...More data for this Ligand-Target Pair
TargetFatty-acid amide hydrolase 1(Human)
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Johnson & Johnson Pharmaceutical Research and Development
Curated by ChEMBL
Affinity DataKi: 2.20E+4nMAssay Description:Binding affinity to soybean LO1More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)