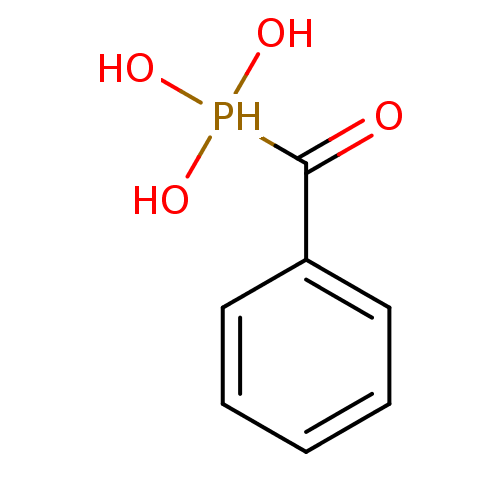
BDBM50112455 (Hydroxy-phenyl-methyl)-phosphonic acid::Alpha Hydroxy benzylphosphonate::CHEMBL24720
SMILES OP(O)(O)C(=O)c1ccccc1
InChI Key InChIKey=XPTRNPPOZCOBEO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50112455
Found 3 hits for monomerid = 50112455
TargetMandelate racemase(Pseudomonas putida (g-Proteobacteria))
Dalhousie University
Curated by ChEMBL
Dalhousie University
Curated by ChEMBL
Affinity DataKi: 4.70E+3nMpH: 7.5Assay Description:Inhibitory activity against Mandelate racemase at pH 7.5 at 25 degree CMore data for this Ligand-Target Pair
TargetMandelate racemase(Pseudomonas putida (g-Proteobacteria))
Dalhousie University
Curated by ChEMBL
Dalhousie University
Curated by ChEMBL
Affinity DataKi: 4.70E+3nMpH: 7.5Assay Description:Inhibition constant against Mandelate racemase from Pseudomonas putida at pH 7.5More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+7nMAssay Description:The compound was evaluated for its binding affinity towards phosphotyrosine binding pocket of Src protein tyrosine kinase SH2 domainMore data for this Ligand-Target Pair