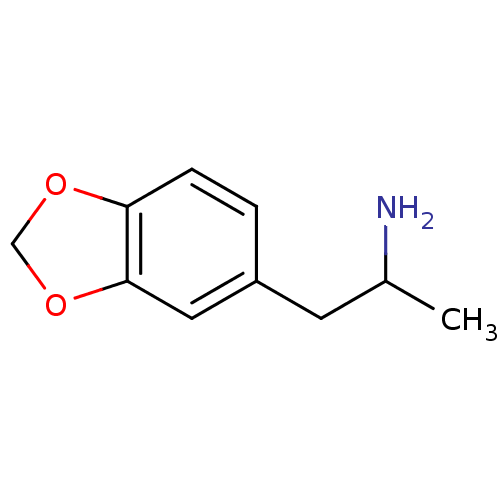
BDBM50005247 (+/-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine::(-)2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine::(R)-(-)-2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine::(S)-(+)-2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine::2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine::2-Benzo[1,3]dioxol-5-yl-1-methyl-ethylamine((R)-(-)-MDA)::3,4-methylenedioxyamphetamine::CHEMBL6731::MDA::MDA, (R,S)::MDA,R(-)::Tenamfetamine::methylenedioxyamphetamine
SMILES CC(N)Cc1ccc2OCOc2c1
InChI Key InChIKey=NGBBVGZWCFBOGO-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 73 hits for monomerid = 50005247
Found 73 hits for monomerid = 50005247
Affinity DataKi: 91nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 98.2nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataEC50: 160nMAssay Description:Activity at SERT in rat synaptosomes assessed as release of [3H]HT after 5 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
TargetSodium-dependent dopamine transporter(Rat)
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
TargetSodium-dependent dopamine transporter(Rat)
Case Western Reserve University
Curated by PDSP Ki Database
Case Western Reserve University
Curated by PDSP Ki Database
Affinity DataEC50: 190nMAssay Description:Activity at DAT in rat synaptosomes assessed as release of [3H]DA after 5 mins by liquid scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataKi: 229nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataIC50: 266nMAssay Description:Compound was tested for the ability to inhibit [3H]norepinephrine binding to Norepinephrine transporter in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataIC50: 266nMAssay Description:Binding affinity to human NETMore data for this Ligand-Target Pair
Affinity DataIC50: 266nMAssay Description:Inhibition of human noradrenaline transporterMore data for this Ligand-Target Pair
Affinity DataEC50: 328nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKd: 355nMAssay Description:Affinity against 5-hydroxytryptamine 2B receptor in the isolated rat stomach fundusMore data for this Ligand-Target Pair
Affinity DataKi: 387nMAssay Description:Displacement of [3H]DOB from 5HT2A in Sprague-Dawley rat cortex membranes measured after 15 mins by scintillation counting methodMore data for this Ligand-Target Pair
Affinity DataIC50: 478nMAssay Description:Inhibition of [3H]5-HT uptake at SERT in rat brain synaptosomeMore data for this Ligand-Target Pair
Affinity DataIC50: 478nMAssay Description:Binding affinity to human SERTMore data for this Ligand-Target Pair
Affinity DataIC50: 478nMAssay Description:Activity towards Serotonin transporter was determined by the ability to inhibit [3H]5-HT uptake in rat synaptosomesMore data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataIC50: 890nMAssay Description:Binding affinity to human DATMore data for this Ligand-Target Pair
Affinity DataIC50: 890nMAssay Description:Inhibition of human dopamine transporterMore data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of [3H]-serotonin reuptake at human SERT expressed in HEK293 cells after 15 to 20 mins by fluorescence neurotransmitter transporter assayMore data for this Ligand-Target Pair
Affinity DataIC50: 996nMAssay Description:Inhibition of [3H]5-HT reuptake at rat SERT expressed in HEK293 cells after 2 mins by liquid scintillation countingMore data for this Ligand-Target Pair
Affinity DataEC50: 1.44E+3nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKi: 1.66E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataEC50: 1.77E+3nMAssay Description:Data from pone.0009019.s006.doc, supplement tables.More data for this Ligand-Target Pair
Affinity DataKi: 2.10E+3nMAssay Description:Compound was tested for binding affinity towards 5-HT1C (5-HT1C) receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using...More data for this Ligand-Target Pair
Affinity DataKi: 2.19E+3nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2 receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using [3H]ketanserin as...More data for this Ligand-Target Pair
Affinity DataKi: 2.19E+3nMAssay Description:Binding affinity towards 5-hydroxytryptamine 2 receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using [3H]ketanserin as...More data for this Ligand-Target Pair
Affinity DataKi: 2.29E+3nMAssay Description:Compound was tested for binding affinity towards 5-HT1C (5-HT1C) receptor from frontal cortical regions of male Sprague-Dawley rat homogenates, using...More data for this Ligand-Target Pair
Affinity DataKi: 3.55E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 3.76E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: 6.42E+3nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Table S2 shows raw Ki data for the current study combined with data collected from the literature for the ten additional compounds.More data for this Ligand-Target Pair