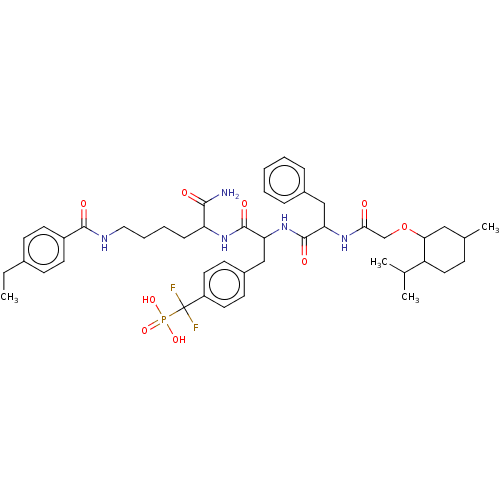
BDBM199180 US9217012, 10
SMILES CCc1ccc(cc1)C(=O)NCCCCC(NC(=O)C(Cc1ccc(cc1)C(F)(F)P(O)(O)=O)NC(=O)C(Cc1ccccc1)NC(=O)COC1CC(C)CCC1C(C)C)C(N)=O
InChI Key InChIKey=DZZMIHDOJVEPRL-UHFFFAOYSA-N
Data 16 KI
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 16 hits for monomerid = 199180
Found 16 hits for monomerid = 199180
TargetTyrosine-protein phosphatase non-receptor type 2(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: 4.10nM ΔG°: -11.4kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 2(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: 4.30nM ΔG°: -11.4kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: 23.1nM ΔG°: -10.4kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 1(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: 34nM ΔG°: -10.2kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 7(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 22(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 13(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 9(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetSerine/threonine-protein phosphatase 2A activator(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase F(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetReceptor-type tyrosine-protein phosphatase C(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase CDC14A(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 3(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetDual specificity protein phosphatase 22(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair
TargetLow molecular weight phosphotyrosine protein phosphatase(Human)
Indiana University Research and Technology
US Patent
Indiana University Research and Technology
US Patent
Affinity DataKi: >1.00E+3nM ΔG°: >-8.18kcal/molepH: 7.0 T: 2°CAssay Description:PTP activity was assayed using p-nitrophenyl phosphate (pNPP) as a substrate in DMG buffer (50 mM DMG, pH 7.0, 1 mM EDTA, 150 mM NaCl, 2 mM DTT, 0.1 ...More data for this Ligand-Target Pair