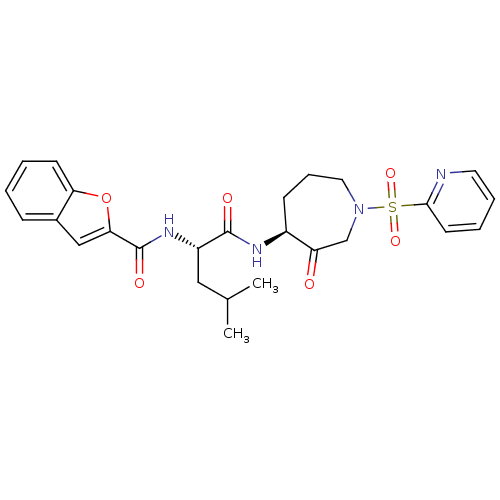
BDBM19769 (2S)-2-(1-benzofuran-2-ylformamido)-4-methyl-N-[(4S)-3-oxo-1-(pyridine-2-sulfonyl)azepan-4-yl]pentanamide::Azepan-3-one compound 1::CHEMBL286364
SMILES CC(C)C[C@H](NC(=O)c1cc2ccccc2o1)C(=O)N[C@H]1CCCN(CC1=O)S(=O)(=O)c1ccccn1
InChI Key InChIKey=VBPPNJCVXGAZDD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 19 hits for monomerid = 19769
Found 19 hits for monomerid = 19769
Affinity DataKi: 0.160nM ΔG°: -13.2kcal/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Inhibition of Human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 0.160nMAssay Description:Apparent inhibitory constant against human cathepsin KMore data for this Ligand-Target Pair
Affinity DataKi: 1.80nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Apparent inhibitory constant against human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 2.20nM ΔG°: -11.7kcal/molepH: 5.5 T: 2°CAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 2.20nMAssay Description:Inhibitory activity against Human cathepsin LMore data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 4nMAssay Description:Inhibitory activity against Human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 4.30nMAssay Description:Apparent inhibitory constant against human cathepsin SMore data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 60nMAssay Description:Inhibitory activity against Rat cathepsin KMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:In vitro inhibitory concentration against cathepsin K in cell-based assay of bone resorptionMore data for this Ligand-Target Pair
Affinity DataIC50: 70nMAssay Description:in vitro cell based assay for measuring the IC50 values of osteoblast resorption.More data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:In situ inhibitory concentration against cathepsin in human tissue sections containing osteoclastsMore data for this Ligand-Target Pair
Affinity DataIC50: 80nMAssay Description:In vitro IC50 value was measured using in situ cytochem assay.More data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Apparent inhibitory constant against human cathepsin BMore data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Potential inhibitors were evaluated using the progress curve method. Assays were carried out in the presence of variable concentrations of test compo...More data for this Ligand-Target Pair
Affinity DataKi: 500nMAssay Description:Inhibitory activity against Human cathepsin BMore data for this Ligand-Target Pair