BDBM126826 US8785467, 1-29
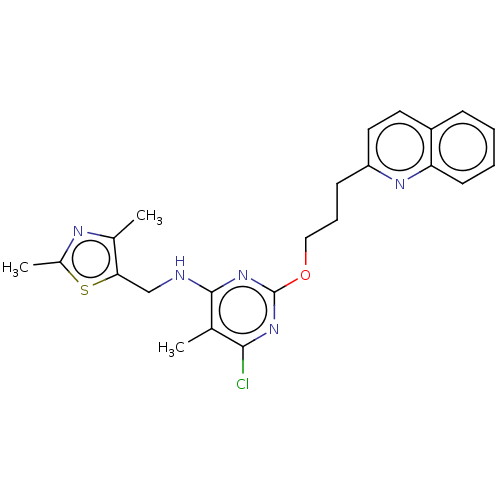
SMILES Cc1c(nc(nc1Cl)OCCCc2ccc3ccccc3n2)NCc4c(nc(s4)C)C
InChI Key InChIKey=DWLPNEAIQATTGD-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 10 hits for monomerid = 126826
Found 10 hits for monomerid = 126826
Affinity DataKi: 0.00800nMAssay Description:Inhibition of human PDE10A2 transfected in human AD293 cells cytosolic fraction using cAMP as substrate by fluorescence polarization assayMore data for this Ligand-Target Pair
Affinity DataKi: 0.00800nMAssay Description:The activity of the compounds in accordance with the present invention as PDE10 inhibitors may be readily determined without undue experimentation us...More data for this Ligand-Target Pair
Affinity DataKi: 0.00820nMAssay Description:Inhibition of human PDE10A2 transfected in AD293 cells by IMAP FP assayMore data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 300nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 900nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 2.00E+3nMAssay Description:Activation of PXR (unknown origin) assessed as CYP3A4 inductionMore data for this Ligand-Target Pair
Affinity DataEC50: 2.30E+3nMAssay Description:Activation of PXR (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+3nMAssay Description:Displacement of MK499 from hERGMore data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)