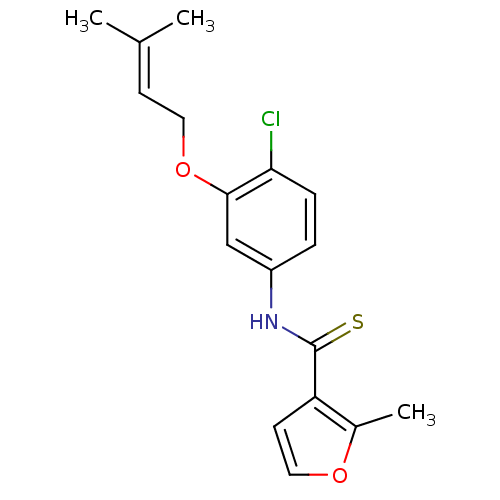
BDBM50105629 CHEMBL54893::N-(4-chloro-3-(3-methylbut-2-enyloxy)phenyl)-2-methylfuran-3-carbothioamide::N-{4-chloro-3-[(3-methylbut-2-enyl)oxy]phenyl}-2-methylfuran-3-carbothioamide::UC-781
SMILES [#6]\[#6](-[#6])=[#6]/[#6]-[#8]-c1cc(-[#7]-[#6](=S)-c2ccoc2-[#6])ccc1Cl
InChI Key InChIKey=JSNDJOKJRHLLQZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50105629
Found 5 hits for monomerid = 50105629
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataEC50: 9nMAssay Description:Effective concentration required against wild type HIV-1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataEC50: 24nMAssay Description:Effective concentration required against L100I mutant HIV-1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 226nMAssay Description:Inhibitory activity against E138K mutant reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 23.8nMAssay Description:Inhibitory activity against wild-type HIV-1 reverse transcriptaseMore data for this Ligand-Target Pair
TargetReverse transcriptase/RNaseH(Human immunodeficiency virus type 1)
Yale University
Curated by ChEMBL
Yale University
Curated by ChEMBL
Affinity DataIC50: 20.8nMAssay Description:Inhibitory activity against R172A mutant reverse transcriptaseMore data for this Ligand-Target Pair