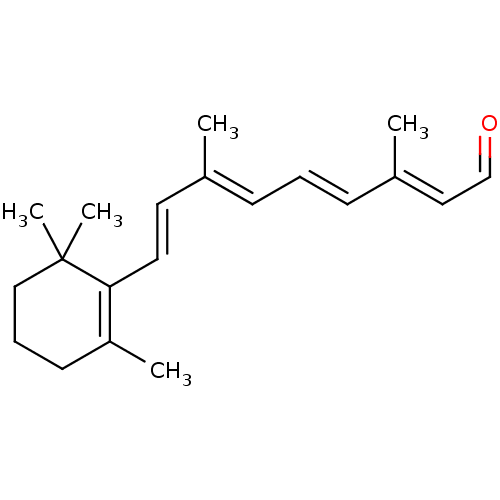
BDBM50553255 All-Trans-Retinal::CHEBI:17898::Retinal
SMILES CC1=C(C(CCC1)(C)C)/C=C/C(=C/C=C/C(=C/C=O)/C)/C
InChI Key InChIKey=NCYCYZXNIZJOKI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50553255
Found 3 hits for monomerid = 50553255
Affinity DataIC50: 180nMAssay Description:Inhibition of amyloid beta (1 to 40) (unknown origin) incubated for 24 hrs to 7 days by thioflavin S based fluorescence assayMore data for this Ligand-Target Pair
Affinity DataIC50: 190nMAssay Description:Inhibition of alpha-synuclein fibril formation (unknown origin) incubated for 24 hrs to 7 days by thioflavin S based fluorescence assayMore data for this Ligand-Target Pair
TargetNuclear receptor subfamily 2 group E member 1(Human)
Goethe University Frankfurt
Curated by ChEMBL
Goethe University Frankfurt
Curated by ChEMBL
Affinity DataEC50: 200nMAssay Description:Inverse agonist activity at TLX (unknown origin)More data for this Ligand-Target Pair