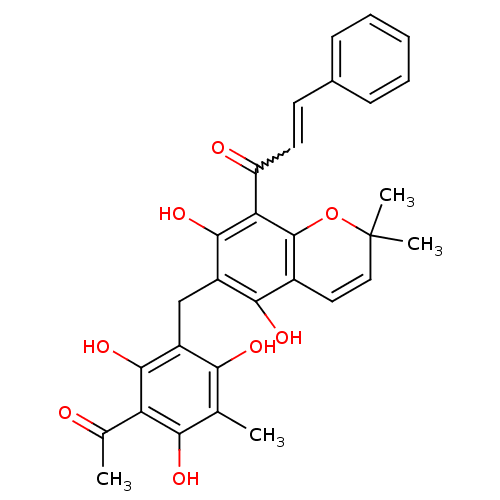
BDBM50126829 (E)-1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl)-3-phenylprop-2-en-1-one::1-(6-(3-acetyl-2,4,6-trihydroxy-5-methylbenzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl)-3-phenylprop-2-en-1-one::1-[6-(3-Acetyl-2,4,6-trihydroxy-5-methyl-benzyl)-5,7-dihydroxy-2,2-dimethyl-2H-chromen-8-yl]-3-phenyl-propenone::CHEMBL34241::R5648 (Rottlerin)::ROTTLERIN
SMILES CC(=O)c1c(O)c(C)c(O)c(Cc2c(O)c3C=CC(C)(C)Oc3c(C(=O)C=Cc3ccccc3)c2O)c1O
InChI Key InChIKey=DEZFNHCVIZBHBI-UHFFFAOYSA-N
Data 14 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 50126829
Found 14 hits for monomerid = 50126829
TargetProtein kinase C alpha type/glutamine gamma-glutamyltransferase 2(Human)
Duke University Medical Center
Duke University Medical Center
Affinity DataIC50: 2.00E+4nMT: 2°CAssay Description:In order to eliminated fluorescence interference, chemicals were subjected to a secondary screening using colorimetric BP incorporation assay. TGase...More data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of His-tagged human PRAK expressed in Sf9 cellsMore data for this Ligand-Target Pair
Affinity DataIC50: 5.40E+3nMAssay Description:Inhibition of His-tagged human MAPKAPK2 expressed in Escherichia coli at 10 uMMore data for this Ligand-Target Pair
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibitory activity against Chymotrypsinogen from Thermus flavusMore data for this Ligand-Target Pair
Affinity DataIC50: 700nMAssay Description:Inhibitory activity against malate dehydrogenase (MDH) from Thermus flavusMore data for this Ligand-Target Pair
Affinity DataIC50: 1.90E+3nMAssay Description:Inhibition of p38-regulated activated kinase (PRAK)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of Protein kinase C delta (PKCdelta)More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+3nMAssay Description:Inhibitory activity against Amp C beta-LactamaseMore data for this Ligand-Target Pair
TargetWD repeat-containing protein 48(Human)
University of Connecticut Health Center
Curated by ChEMBL
University of Connecticut Health Center
Curated by ChEMBL
Affinity DataIC50: 8.00E+3nMAssay Description:Inhibition of human USP1/UAF1 complex using Ub-Rho as substrate by qHTS assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.42E+5nMAssay Description:Inhibition of Enterobacter cloacae beta-lactamase incubated for 10 mins followed by nitrocefin substrate challenge and measured for 5 mins in presenc...More data for this Ligand-Target Pair
Affinity DataIC50: 9.60E+4nMAssay Description:Inhibition of Enterobacter cloacae beta-lactamase incubated for 10 mins followed by nitrocefin substrate challenge and measured for 5 mins by spectro...More data for this Ligand-Target Pair
Affinity DataIC50: 5.30E+3nMAssay Description:Inhibition of eEF2K (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 3.00E+3nMAssay Description:Inhibition of PKCdelta (unknown origin) by IMAP kinase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Inhibition of RUVBL1 (unknown origin)More data for this Ligand-Target Pair