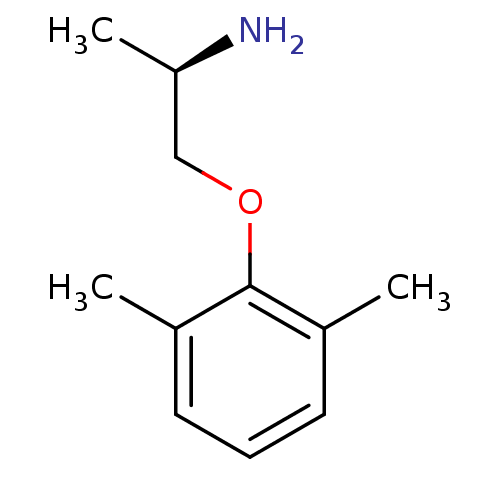
BDBM50135883 (2R)-1-(2,6-dimethylphenoxy)propan-2-amine::(R)-1-(2,6-dimethylphenoxy)propan-2-amine::(R)-2-(2,6-Dimethyl-phenoxy)-1-methyl-ethylamine::CHEMBL147507::MEXILETINE
SMILES C[C@@H](N)COc1c(C)cccc1C
InChI Key InChIKey=VLPIATFUUWWMKC-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50135883
Found 3 hits for monomerid = 50135883
TargetSodium channel protein type 4 subunit alpha(Human)
University of Bari Aldo Moro
Curated by ChEMBL
University of Bari Aldo Moro
Curated by ChEMBL
Affinity DataEC50: 2.29E+4nMAssay Description:Inhibition of Nav1.4 ion channel assessed as reduction in cumulative sodium currentMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of uPAMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+6nMAssay Description:Inhibition of uPAMore data for this Ligand-Target Pair