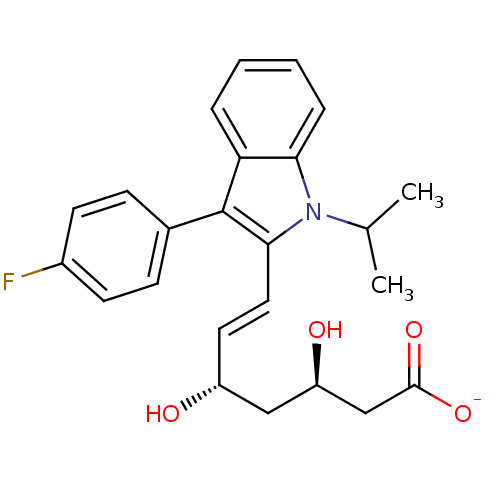
BDBM50368147 (+)-(3R,5S)-fluvastatin::(3R,5S)-fluvastatin::(3R,5S,6E)-7-[3-(4-fluorophenyl)-1-(propan-2-yl)-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid::(3R,5S,6E)-7-[3-(4-fluorophenyl)-1-isopropyl-1H-indol-2-yl]-3,5-dihydroxyhept-6-enoic acid::CHEMBL1078::FLUVASTATIN SODIUM::Fluvastatin::cid_23663976
SMILES CC(C)n1c(\C=C\[C@@H](O)C[C@@H](O)CC([O-])=O)c(-c2ccc(F)cc2)c2ccccc12
InChI Key InChIKey=FJLGEFLZQAZZCD-UHFFFAOYSA-M
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 50368147
Found 6 hits for monomerid = 50368147
TargetProbable global transcription activator SNF2L2(Human)
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
Affinity DataIC50: 2.5nMAssay Description:In vitro inhibition of solubilized HMG-CoA reductase in rat liver.More data for this Ligand-Target Pair
Affinity DataIC50: 2.5nMAssay Description:Inhibitory activity against partially purified rat liver HMG-CoA reductase in vitro; 0.0015-0.0040More data for this Ligand-Target Pair
Affinity DataIC50: 32nMAssay Description:Ability to inhibit microsomal preparation of HMG-CoA reductase in rat liver.More data for this Ligand-Target Pair
Target3-hydroxy-3-methylglutaryl-coenzyme A reductase(Human)
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
National Institute of Pharmaceutical Education and Research (NIPER)
Curated by ChEMBL
Affinity DataIC50: 28nMAssay Description:Inhibitory concentration against 3-hydroxy-3-methylglutaryl-CoA reductaseMore data for this Ligand-Target Pair
Affinity DataIC50: 3nMAssay Description:Inhibition of rat HMG-CoA reductase using 0.37 MBq DL-[3-14C]HMG-CoAMore data for this Ligand-Target Pair