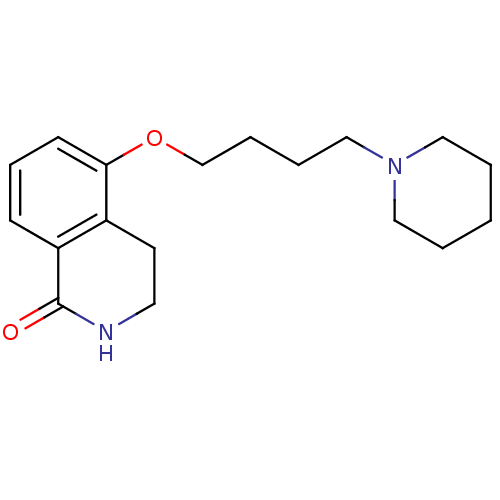
BDBM27502 3,4-dihydro-5-(4-(1-piperidinyl)butoxy)-1(2H)-isoquinolinone::5-[4-(piperidin-1-yl)butoxy]-1,2,3,4-tetrahydroisoquinolin-1-one::CHEMBL127336::DPQ
SMILES c1cc2c(c(c1)OCCCCN3CCCCC3)CCNC2=O
InChI Key InChIKey=RVOUDNBEIXGHJY-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 6 hits for monomerid = 27502
Found 6 hits for monomerid = 27502
Affinity DataIC50: 53nMAssay Description:Inhibition of PARP1 (unknown origin) in presence of NAD+ by homogenous fluorescence plate assayMore data for this Ligand-Target Pair
Affinity DataIC50: 150nMAssay Description:Inhibition of PARP1 (unknown origin)More data for this Ligand-Target Pair
Affinity DataIC50: 372nMpH: 7.8 T: 2°CAssay Description:The enzymatic reaction of the recombinant PARP was quantified by SPA. Radioactivity incorporated from [3H]NAD+ into PAR, and then being captured by P...More data for this Ligand-Target Pair
TargetTransient receptor potential cation channel subfamily M member 2(Human)
Queen'S Center For Biomedical Research
Curated by ChEMBL
Queen'S Center For Biomedical Research
Curated by ChEMBL
Affinity DataIC50: 600nMAssay Description:Inhibition of human FLAG-tagged TRPM2 expressed in HEK293 cells assessed as reduction in H2O2-induced intracellular calcium flux pretreated for 30 se...More data for this Ligand-Target Pair
Affinity DataIC50: 1.74E+3nMpH: 7.8 T: 2°CAssay Description:The enzymatic reaction of the recombinant PARP was quantified by SPA. Radioactivity incorporated from [3H]NAD+ into PAR, and then being captured by P...More data for this Ligand-Target Pair
Affinity DataIC50: 1.08E+4nMAssay Description:Inhibition of PARP1More data for this Ligand-Target Pair