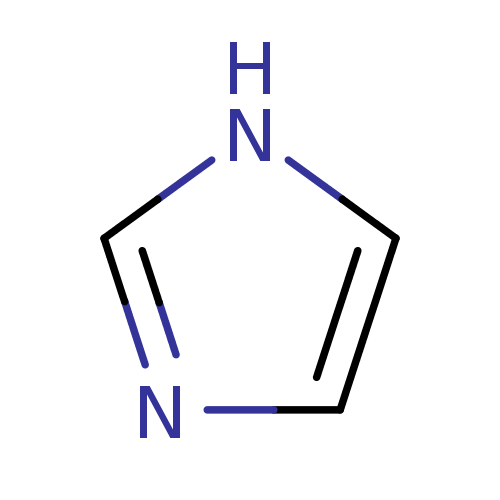
BDBM7882 1H-imidazole::CHEMBL540::Imidazole (Im)::US9138393, Imidazole::US9144538, Imidazole::imidazole
SMILES c1c[nH]cn1
InChI Key InChIKey=RAXXELZNTBOGNW-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 15 hits for monomerid = 7882
Found 15 hits for monomerid = 7882
Affinity DataIC50: 1.00E+4nMAssay Description:A commercially available P450-GLO Assay kit (Promega Corporation, Madison Wis.) is used to screen various compounds for CYP3A4A inhibition activity. ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+4nMAssay Description:Cytochrome P450 is a large and diverse group of enzymes that catalyze the oxidation of organic substances. Some members of the CYP family contribute ...More data for this Ligand-Target Pair
TargetHistamine H3 receptor(Human)
R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
R. W. Johnson Pharmaceutical Research Institute
Curated by PDSP Ki Database
Affinity DataIC50: 4.00E+4nMAssay Description:Inhibition of purified mouse inducible nitric oxide synthase catalyzed [14C]L-citrulline production at a compound concentration of 1 mM in presence o...More data for this Ligand-Target Pair
Affinity DataKd: 5.20E+4nMpH: 7.0Assay Description:Samples for solution resonance Raman spectroscopic studies were prepared withfinal concentrations of 75 μM WT DHP B and 3.75 mM azole in 100 mM ...More data for this Ligand-Target Pair
Affinity DataKi: 1.00E+5nM ΔG°: -5.54kcal/molepH: 8.0 T: 2°CAssay Description:QC activity was evaluated fluorometrically using Gln-AMC as substrate, and pyroglutamyl peptidase as the auxiliary enzyme. After conversion of Gln-AM...More data for this Ligand-Target Pair
Affinity DataKi: 1.60E+5nM ΔG°: -5.26kcal/molepH: 8.0 T: 2°CAssay Description:QC activity was evaluated fluorometrically using Gln-AMC as substrate, and pyroglutamyl peptidase as the auxiliary enzyme. After conversion of Gln-AM...More data for this Ligand-Target Pair
Affinity DataKi: 1.75E+5nMAssay Description:Inhibition of nNOS assessed as conversion of L-[3H]arginine to L-[3H]citrullineMore data for this Ligand-Target Pair
Affinity DataIC50: 3.52E+5nMAssay Description:Inhibition of recombinant human QC using H-Gln-AMC hydrobromide as fluorogenic substrate incubated for 6 hrs by fluorometric microplate reader analys...More data for this Ligand-Target Pair
Affinity DataIC50: 6.70E+5nMAssay Description:Inhibitory activity against thromboxane A2 synthetaseMore data for this Ligand-Target Pair
Affinity DataIC50: 4.00E+6nMAssay Description:Inhibition of porcine aorta prostacyclin PGI-2 synthase by bioassay methodMore data for this Ligand-Target Pair
Affinity DataKd: 4.40E+6nMAssay Description:Binding affinity to 15N-labeled FKBP51 (1 to 140 residues) (unknown origin) expressed in Escherichia coli OD2N by two-dimensional 1H/15N HSQC NMR spe...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+7nMAssay Description:Inhibition of ram seminal vesicle CyclooxygenaseMore data for this Ligand-Target Pair