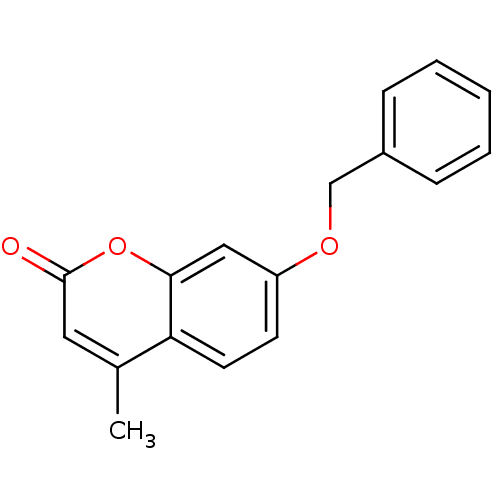
BDBM76662 4-methyl-7-phenylmethoxy-1-benzopyran-2-one::4-methyl-7-phenylmethoxy-chromen-2-one::4-methyl-7-phenylmethoxychromen-2-one::7-Benzyloxy-4-methyl-chromen-2-one::7-benzoxy-4-methyl-coumarin::MLS001212241::SMR000514539::cid_728520
SMILES Cc1cc(=O)oc2cc(OCc3ccccc3)ccc12
InChI Key InChIKey=RTORNQDWFDEDPT-UHFFFAOYSA-N
Data 14 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 14 hits for monomerid = 76662
Found 14 hits for monomerid = 76662
Affinity DataIC50: 1.58nMAssay Description:Inhibition of human supersomes MAOBMore data for this Ligand-Target Pair
Affinity DataIC50: 2nMAssay Description:Inhibition of recombinant human MAO-B assessed as reduction in 4-hydroxyquinolone production using kynuramine as substrate after 20 mins by fluoresce...More data for this Ligand-Target Pair
Affinity DataIC50: 18nMAssay Description:Inhibitory activity against monoamine oxidase BMore data for this Ligand-Target Pair
Affinity DataIC50: 18.2nMAssay Description:Inhibitory effect on Monoamine oxidase B, SD on IC50 values < 10%More data for this Ligand-Target Pair
Affinity DataIC50: 18.2nMAssay Description:Inhibition of rat brain MAOBMore data for this Ligand-Target Pair
Affinity DataIC50: 1.95E+3nMAssay Description:Inhibitory effect on monoamine oxidase A, SD on IC50 values < 10%More data for this Ligand-Target Pair
Affinity DataIC50: 1.95E+3nMAssay Description:Inhibitory activity against monoamine oxidase AMore data for this Ligand-Target Pair
Affinity DataIC50: 2.62E+3nMAssay Description:Inhibition of recombinant human MAO-A assessed as reduction in 4-hydroxyquinolone production using kynuramine as substrate after 20 mins by fluoresce...More data for this Ligand-Target Pair
TargetTyrosyl-DNA phosphodiesterase 1(Human)
Siberian Branch of The Russian Academy of Sciences
Curated by ChEMBL
Siberian Branch of The Russian Academy of Sciences
Curated by ChEMBL
Affinity DataIC50: 5.28E+3nMAssay Description:Inhibition of recombinant Tdp1 (unknown origin) using 5'-(5,6 FAM-AAC GTC AGG GTC TTC C-BHQ1)-3' as substrate measured every 1 min by fluorescence an...More data for this Ligand-Target Pair
Affinity DataIC50: 9.60E+3nMAssay Description:Inhibition of equine serum BuChE using S-butylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured after ...More data for this Ligand-Target Pair
Affinity DataIC50: 1.65E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetSentrin-specific protease 8(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.10E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetSentrin-specific protease 6(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 2.41E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition measured after 10...More data for this Ligand-Target Pair