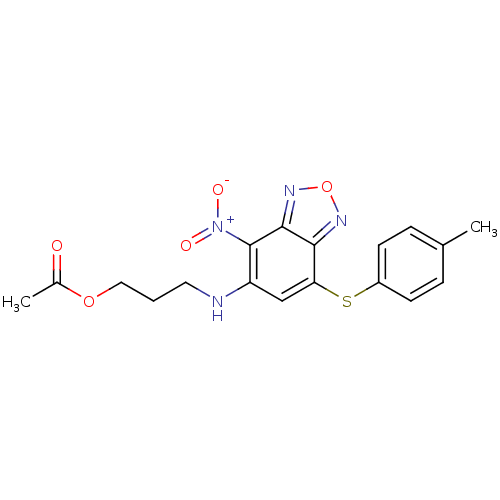
BDBM63210 3-({7-[(4-methylphenyl)thio]-4-nitro-2,1,3-benzoxadiazol-5-yl}amino)propyl acetate::3-[[7-(4-methylphenyl)sulfanyl-4-nitro-2,1,3-benzoxadiazol-5-yl]amino]propyl acetate::3-[[7-(4-methylphenyl)sulfanyl-4-nitro-2,1,3-benzoxadiazol-5-yl]amino]propyl ethanoate::MLS000584455::SMR000207041::acetic acid 3-[[4-nitro-7-(p-tolylthio)benzofurazan-5-yl]amino]propyl ester::acetic acid 3-[[7-[(4-methylphenyl)thio]-4-nitro-2,1,3-benzoxadiazol-5-yl]amino]propyl ester::cid_12005285
SMILES CC(=O)OCCCNc1cc(Sc2ccc(C)cc2)c2nonc2c1[N+]([O-])=O
InChI Key InChIKey=KRGXSGJOUWFLKQ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 63210
Found 4 hits for monomerid = 63210
TargetType-1 angiotensin II receptor(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 1.56E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute(SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.84E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford- Sanford-Burnham Medical Research Institute(SBMRI, San...More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 5.83E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: >1.95E+5nMAssay Description:Keywords: Heat Shock Factor-1 (HSF-1), Stress Response, MG132, NIH3T3, Luminescence Assay Overview: Modified NIH3T3, transformed to express firefly...More data for this Ligand-Target Pair