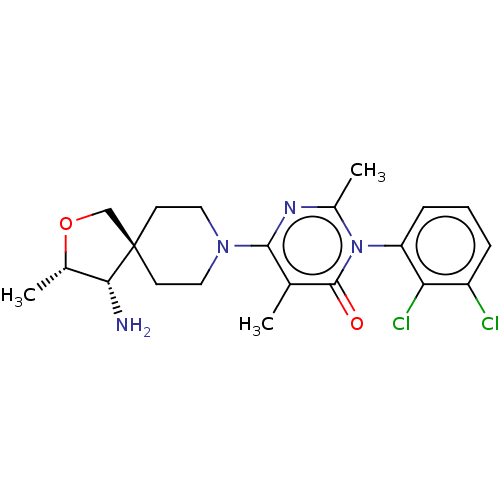
BDBM608845 (3M)-6-[(3S,4S)-4-amino-3- methyl-2-oxa-8- azaspiro[4.5]decan-8-yl]-3-(2,3- dichlorophenyl)-2,5-dimethyl-3,4- dihydropyrimidin-4-one::US11702392, Compound 23b
SMILES C[C@@H]1OC[C@]2(CCN(CC2)c2nc(C)n(-c3cccc(Cl)c3Cl)c(=O)c2C)[C@@H]1N
InChI Key InChIKey=ABKATOFAZUIEJX-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 608845
Found 5 hits for monomerid = 608845
Affinity DataIC50: 9nMAssay Description:The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing...More data for this Ligand-Target Pair
Affinity DataIC50: 12nMAssay Description:The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing...More data for this Ligand-Target Pair
Affinity DataIC50: 21nMAssay Description:The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing...More data for this Ligand-Target Pair
Affinity DataKi: >1.00E+4nMAssay Description:Inhibition of the ion channel hErg (or Kv11.1) current causes QT interval prolongation resulting in potentially fatal ventricular tachyarrhythmia cal...More data for this Ligand-Target Pair
Affinity DataIC50: 1.20E+4nMAssay Description:The inhibition of SHP2 by compounds of the invention was monitored using the surrogate substrate DiFMUP after protein activation by a peptide bearing...More data for this Ligand-Target Pair