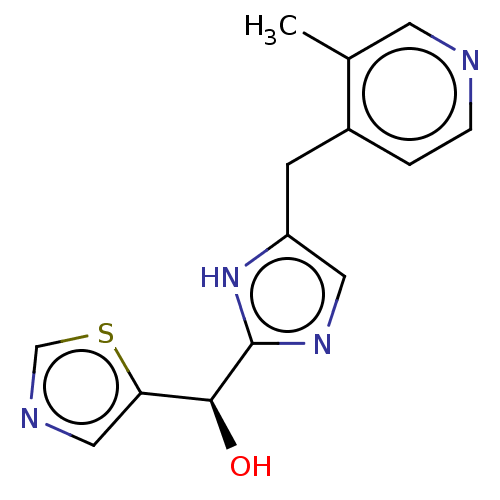
BDBM600594 (S)-(5-((3-Methylpyridin-4-yl)methyl)-1H-imidazol-2-yl)(thiazol-5-yl)methanol and (R)-(5-((3-methylpyridin-4-yl)methyl)-1H-imidazol-2-yl)(thiazol-5-yl)methanol::US11629136, Example 30::US11629136, Example 30a
SMILES Cc1cnccc1Cc1cnc([nH]1)[C@@H](O)c1cncs1
InChI Key InChIKey=UVBHRIDAJWKMAG-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 600594
Found 2 hits for monomerid = 600594
Affinity DataIC50: 5.50E+3nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair
Affinity DataIC50: 5.50E+4nMAssay Description:Each reaction was run at a volume of 20 μL containing 50 μM compound (dissolved in DMSO; final concentration of DMSO is 1% v/v), 40 nM huma...More data for this Ligand-Target Pair