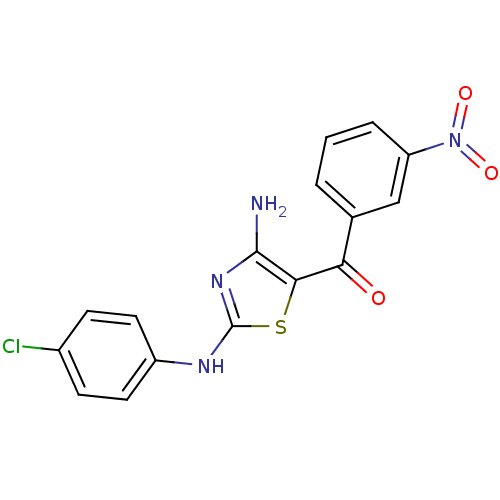
BDBM59194 4-aminothiazole, 2.0
SMILES c1cc(cc(c1)[N+](=O)[O-])C(=O)c2c(nc(s2)Nc3ccc(cc3)Cl)N
InChI Key InChIKey=YQRVBHMYUSGXHL-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 59194
Found 2 hits for monomerid = 59194
Affinity DataKi: 660nM IC50: 2.00E+3nMAssay Description:Cdk5, 33P-ATP and cofactors were added in the presence of tau protein. The reaction mixture was incubated to allow Cdk5 to transfer 33P from ATP to ...More data for this Ligand-Target Pair
Affinity DataKi: 690nMAssay Description:Cdk5, 33P-ATP and cofactors were added in the presence of tau protein. The reaction mixture was incubated to allow Cdk5 to transfer 33P from ATP to ...More data for this Ligand-Target Pair
 3D Structure (crystal)
3D Structure (crystal)