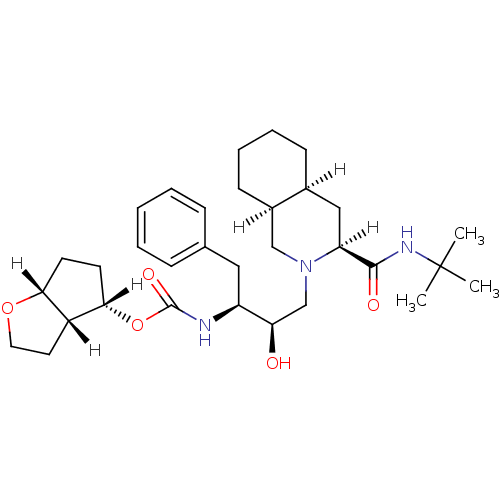
BDBM568 (3S,4aS,8aS,2 R,3 S,3 aS,4 S,6 aR)-N-tert-Butyl-2-[2 -hydroxy-4 -phenyl-3 -[[[(4 -hexahydro-2H-cyclopenta[b]-furanyl)oxy]carbonyl]amino]butyl]decahydroiso-quinoline-3-carboxamide::(3aR,4S,6aR)-hexahydro-2H-cyclopenta[b]furan-4-yl N-[(2S,3R)-4-[(3S,4aS,8aS)-3-(tert-butylcarbamoyl)-decahydroisoquinolin-2-yl]-3-hydroxy-1-phenylbutan-2-yl]carbamate::Isoquinoline furanyl urethane analog. 8
SMILES [H][C@@]1(CC[C@@]2([H])OCC[C@@]12[H])OC(=O)N[C@@H](Cc1ccccc1)[C@H](O)CN1C[C@@]2([H])CCCC[C@@]2([H])C[C@@]1([H])C(=O)NC(C)(C)C
InChI Key InChIKey=BXHKUQJOWIKKIH-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 568
Found 2 hits for monomerid = 568
TargetDimer of Gag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
University of Illinois At Chicago
University of Illinois At Chicago
Affinity DataIC50: 17nMpH: 5.5 T: 2°CAssay Description:The IC50 values for the compounds were determined using purified HIV-1 Protease. Inhibition of the cleavage of the peptide H-Val-Ser-Gln-Asn-(L-beta-...More data for this Ligand-Target Pair
TargetGag-Pol polyprotein [489-587](Human immunodeficiency virus type 1)
Bristol Myers Squibb Research and Early Development
Curated by ChEMBL
Bristol Myers Squibb Research and Early Development
Curated by ChEMBL
Affinity DataIC50: 17nMAssay Description:Inhibition of HIV-1 proteaseMore data for this Ligand-Target Pair