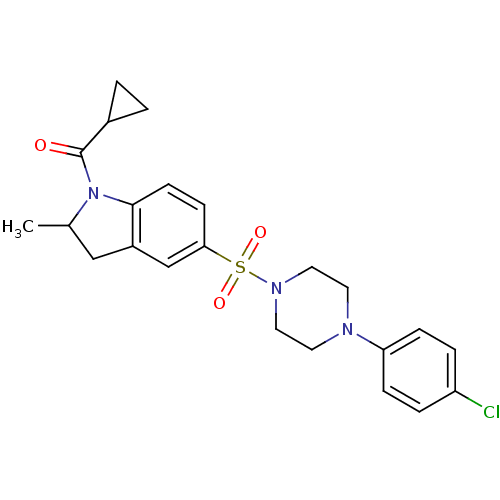
BDBM54502 CHEMBL1351584::MLS001122908::SMR000628306::[5-[4-(4-chlorophenyl)piperazin-1-yl]sulfonyl-2-methyl-2,3-dihydroindol-1-yl]-cyclopropyl-methanone::[5-[4-(4-chlorophenyl)piperazin-1-yl]sulfonyl-2-methyl-2,3-dihydroindol-1-yl]-cyclopropylmethanone::[5-[4-(4-chlorophenyl)piperazino]sulfonyl-2-methyl-indolin-1-yl]-cyclopropyl-methanone::[5-[[4-(4-chlorophenyl)-1-piperazinyl]sulfonyl]-2-methyl-2,3-dihydroindol-1-yl]-cyclopropylmethanone::cid_20901603
SMILES CC1Cc2cc(ccc2N1C(=O)C1CC1)S(=O)(=O)N1CCN(CC1)c1ccc(Cl)cc1
InChI Key InChIKey=XVBXKCJNSJIPOJ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 7 hits for monomerid = 54502
Found 7 hits for monomerid = 54502
Affinity DataIC50: 2.22E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 3.63E+3nMAssay Description:Inhibition of NOD-2 mediated NFkappaB activation in HEK293T cells assessed as inhibition of MDP-induced luciferase activity after 14 hrs by reporter ...More data for this Ligand-Target Pair
Affinity DataIC50: 4.43E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Inhibition of NOD-1 mediated NFkappaB activation in HEK293T cells assessed as inhibition of gamma-tri-DAP-induced luciferase activity after 14 hrs by...More data for this Ligand-Target Pair
Affinity DataIC50: 5.60E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataIC50: 9.52E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 2.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair