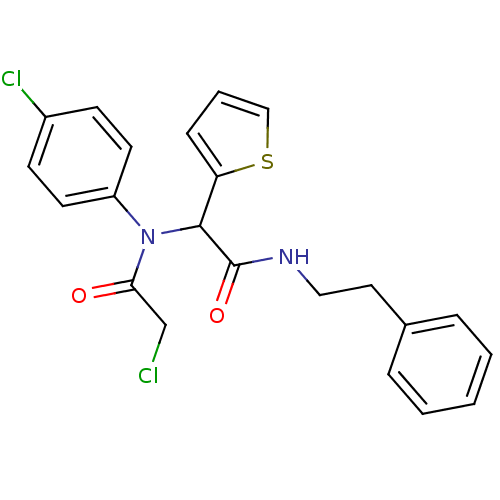
BDBM54238 2-(4-chloro-N-(2-chloro-1-oxoethyl)anilino)-N-(2-phenylethyl)-2-thiophen-2-ylacetamide::2-(4-chloro-N-(2-chloroacetyl)anilino)-N-(2-phenylethyl)-2-thiophen-2-ylacetamide::2-(4-chloro-N-(2-chloroacetyl)anilino)-N-phenethyl-2-(2-thienyl)acetamide::2-[(chloroacetyl)(4-chlorophenyl)amino]-N-(2-phenylethyl)-2-thien-2-ylacetamide::2-[2-chloranylethanoyl-(4-chlorophenyl)amino]-N-(2-phenylethyl)-2-thiophen-2-yl-ethanamide::MLS000583954::SMR000206940::cid_3689411
SMILES ClCC(=O)N(C(C(=O)NCCc1ccccc1)c1cccs1)c1ccc(Cl)cc1
InChI Key InChIKey=IMWYYSDFZCXBLG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 9 hits for monomerid = 54238
Found 9 hits for monomerid = 54238
Affinity DataEC50: 1.28E+3nMAssay Description:Keywords: Heat Shock Factor-1 (HSF-1), Stress Response, MG132, NIH3T3, Luminescence Assay Overview: Modified NIH3T3, transformed to express firefly...More data for this Ligand-Target Pair
TargetNeurotensin receptor type 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 2.50E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetPlatelet-derived growth factor receptor alpha/beta(Mouse)
Indiana University School of Medicine 635 Barnhill Drive
Curated by ChEMBL
Indiana University School of Medicine 635 Barnhill Drive
Curated by ChEMBL
Affinity DataIC50: 4.00E+3nMAssay Description:Inhibition of GST-tagged TEAD4 (217 to 434 residues) (unknown origin) expressed in Escherichia coli BL21 (DE3) cells/FAM-labelled YAP1 (60 to 99 resi...More data for this Ligand-Target Pair
Affinity DataIC50: 4.24E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetNucleotide-binding oligomerization domain-containing protein 1(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataEC50: 5.30E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetNucleotide-binding oligomerization domain-containing protein 2(Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 5.36E+3nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
Affinity DataEC50: 7.95E+3nMAssay Description:Keywords: apoptosis, BH3 domain, Bcl2-A1, BIM, caspase, cancer Primary Collaborator: Todd Golub, Broad Institute, golub@broadinstitute.org Assay Over...More data for this Ligand-Target Pair
TargetHexokinase HKDC1 [W721R](Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 3.57E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair
TargetHexokinase HKDC1 [W721R](Human)
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Burnham Center For Chemical Genomics
Curated by PubChem BioAssay
Affinity DataIC50: 8.00E+4nMAssay Description:Data Source: Sanford-Burnham Center for Chemical Genomics (SBCCG) Source Affiliation: Sanford-Burnham Medical Research Institute (SBMRI, San Diego, C...More data for this Ligand-Target Pair