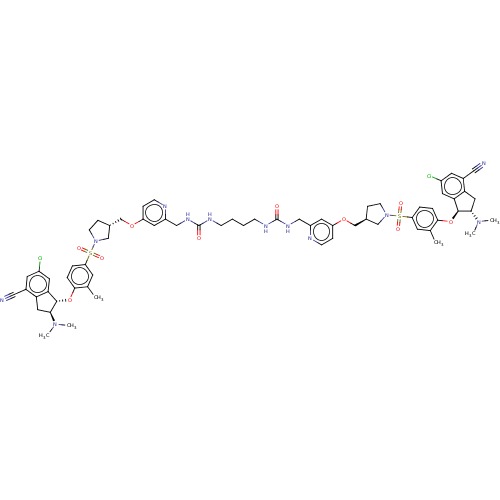
BDBM535943 US11242337, Example 50
SMILES CN(C)[C@H]1Cc2c(cc(Cl)cc2C#N)[C@@H]1Oc1ccc(cc1C)S(=O)(=O)N1CC[C@H](COc2ccnc(CNC(=O)NCCCCNC(=O)NCc3cc(OC[C@H]4CCN(C4)S(=O)(=O)c4ccc(O[C@@H]5[C@H](Cc6c5cc(Cl)cc6C#N)N(C)C)c(C)c4)ccn3)c2)C1
InChI Key InChIKey=MNTQGKROTBWSCZ-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 535943
Found 2 hits for monomerid = 535943
Affinity DataIC50: 100nMAssay Description:Rat and human NHE-3-mediated Na+-dependent H+ antiport was measured using a modification of the pH sensitive dye method originally reported by Paradi...More data for this Ligand-Target Pair
Affinity DataIC50: 100nMAssay Description:The ability of compounds to inhibit human and rat NHE-3-mediated Na+ dependent H+ antiport after application and washout was measured using a modific...More data for this Ligand-Target Pair