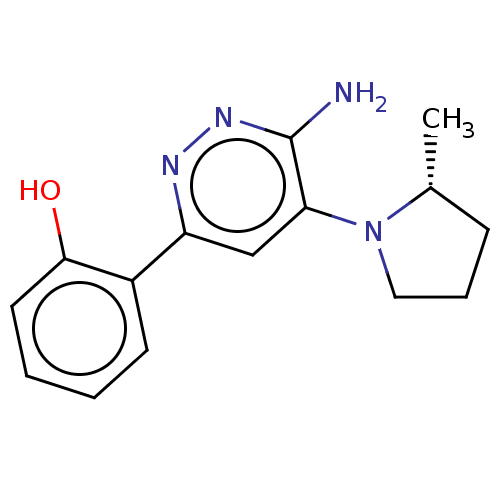
BDBM50628859 CHEMBL5425250
SMILES C[C@@H]1CCCN1c1cc(nnc1N)-c1ccccc1O
InChI Key InChIKey=CMJYPZQQLQOFCI-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50628859
Found 11 hits for monomerid = 50628859
Affinity DataKd: 53nMAssay Description:Binding affinity to PBRM1 bromodomain 5 (unknown origin) assessed as dissociation constant by BROMOscan LeadHunter assayMore data for this Ligand-Target Pair
Affinity DataKd: 340nMAssay Description:Binding affinity to PBRM1 bromodomain 2 (unknown origin) assessed as dissociation constant by BROMOscan LeadHunter assayMore data for this Ligand-Target Pair
Affinity DataKd: 32nMAssay Description:Binding affinity to SMARCA2 (unknown origin) assessed as dissociation constant by BROMOscan LeadHunter assayMore data for this Ligand-Target Pair
Affinity DataKd: 33nMAssay Description:Binding affinity to SMARCA4 (unknown origin) assessed as dissociation constant by BROMOscan LeadHunter assayMore data for this Ligand-Target Pair
Affinity DataIC50: 4.30E+3nMAssay Description:Binding affinity to PXR (unknown origin) by Lanthascreen TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Binding affinity to LXR alpha (unknown origin) by Lanthascreen TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Binding affinity to LXRbeta (unknown origin) by Lanthascreen TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Binding affinity to RXR alpha (unknown origin) by Lanthascreen TR-FRET assayMore data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at PPAR delta in GeneBLAzer PPARdelta-UAS-bla HEK 293T cells preincubated with compound for 16 to 24 hrs followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataEC50: >2.00E+4nMAssay Description:Agonist activity at PPAR gamma in GeneBLAzer PPARgamma-UAS-bla HEK 293H cells preincubated with compound for 16 to 24 hrs followed by substrate addit...More data for this Ligand-Target Pair
Affinity DataEC50: >1.00E+4nMAssay Description:Agonist activity at RARalpha in GeneBLAzer RARalpha-UAS-bla HEK 293T cells preincubated with compound for 16 to 24 hrs followed by substrate addition...More data for this Ligand-Target Pair