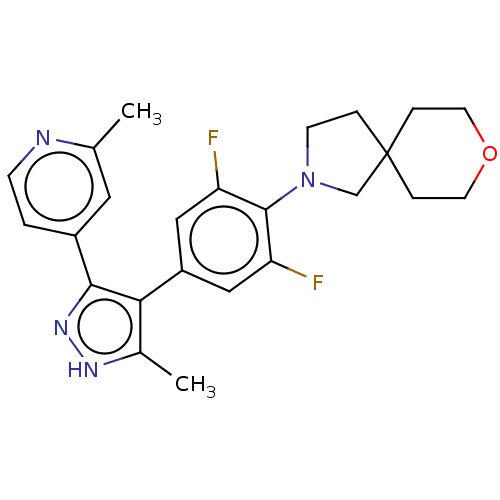
BDBM50624636 CHEMBL5424518
SMILES Cc1[nH]nc(c1-c1cc(F)c(N2CCC3(C2)CCOCC3)c(F)c1)-c1ccnc(C)c1
InChI Key InChIKey=XBRLZBYMEGPKHS-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50624636
Found 4 hits for monomerid = 50624636
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataIC50: 43nMAssay Description:Inhibition of wildtype full length LRRK2 (unknown origin)More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataIC50: 13nMAssay Description:Inhibition of LRRK2 G2019S mutant (unknown origin)More data for this Ligand-Target Pair
Ligand InfoSimilars
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataEC50: 6.23E+3nMAssay Description:Inhibition of wildtype full length LRRK2 in HEK293 cellsMore data for this Ligand-Target Pair
Ligand InfoSimilars
TargetLeucine-rich repeat serine/threonine-protein kinase 2(Human)
Stanford University
Curated by ChEMBL
Stanford University
Curated by ChEMBL
Affinity DataEC50: 490nMAssay Description:Inhibition of LRRK2 G2019S mutant in HEK293 cellsMore data for this Ligand-Target Pair
Ligand InfoSimilars