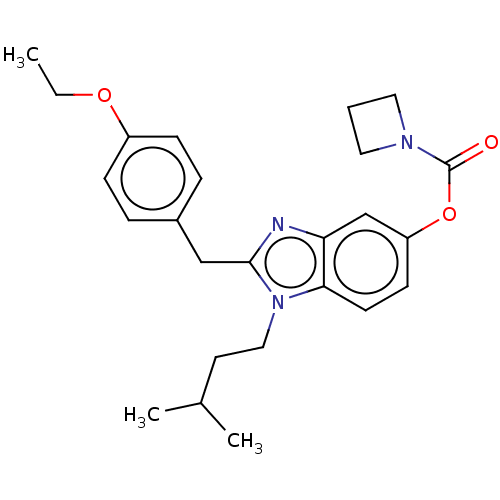
BDBM50621822 CHEMBL5422050
SMILES CCOc1ccc(Cc2nc3cc(OC(=O)N4CCC4)ccc3n2CCC(C)C)cc1
InChI Key InChIKey=ZJWOQJYRYQBQGK-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 5 hits for monomerid = 50621822
Found 5 hits for monomerid = 50621822
Affinity DataIC50: 150nMAssay Description:Pseudo-irreversible inhibition of human BChE using butyrylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ell...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 2.50E+4nMAssay Description:Pseudo-irreversible inhibition of human AChE using acetylthiocholine iodide as substrate incubated for 20 mins followed by substrate addition by Ellm...More data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataIC50: 1.02E+3nMAssay Description:Displacement of [3H]CP55940 from human CB2 receptor expressed in HEK293 cell membrane incubated for 3 hrs by scintillation counter analysisMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataEC50: 1.30E+3nMAssay Description:Agonist activity at human CB2 receptor stably expressed in CHO-K1 cells co-expressing Galphaq16 by Fluo-4AM dye based calcium mobilization assayMore data for this Ligand-Target Pair
Ligand InfoSimilars
Affinity DataEC50: 43nMAssay Description:Agonist activity at human CB2 receptor stably expressed in HEK293 cells assessed as induction of beta-arrestin 2 recruitment incubated for 2 hrs by l...More data for this Ligand-Target Pair
Ligand InfoSimilars