BDBM50620480 CHEMBL5399275
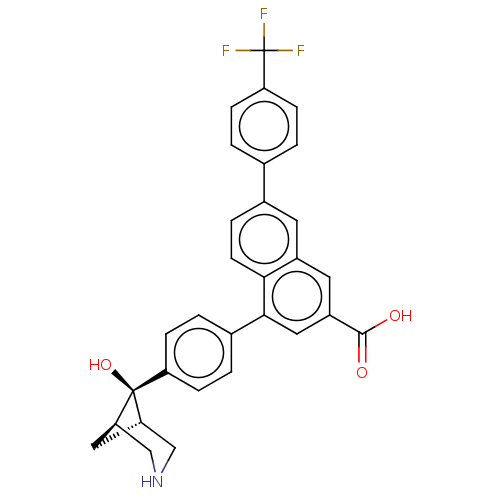
SMILES [H][C@]12C[C@]([H])(CNC1)[C@]2(O)c1ccc(cc1)-c1cc(cc2cc(ccc12)-c1ccc(cc1)C(F)(F)F)C(O)=O
InChI Key InChIKey=BFIYEIMEFITOEG-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 11 hits for monomerid = 50620480
Found 11 hits for monomerid = 50620480
TargetP2Y purinoceptor 14(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 5.90nMAssay Description:Displacement of fluorescent tracer 4-(4-(1-(4-(1-(6-(3-carboxylato-4-(3-iminio-3H-xanthen-9-yl)benzamido)hexyl)-4,5-dihydro-1H-pyrrol-3-yl)butyl)pipe...More data for this Ligand-Target Pair
TargetP2Y purinoceptor 14(Mouse)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 4.80nMAssay Description:Displacement of fluorescent tracer 4-(4-(1-(4-(1-(6-(3-carboxylato-4-(3-iminio-3H-xanthen-9-yl)benzamido)hexyl)-4,5-dihydro-1H-pyrrol-3-yl)butyl)pipe...More data for this Ligand-Target Pair
TargetCytochrome P450 1A2(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP1A2 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C9(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C9 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2C19(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2C19 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 2D6(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP2D6 (unknown origin)More data for this Ligand-Target Pair
TargetCytochrome P450 3A4(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of CYP3A4 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of human ERG stably expressed in human HEK293 cells incubated for 2 hrs by TAMRA based fuorescence polarization assayMore data for this Ligand-Target Pair
TargetP2Y purinoceptor 14(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 3.40nMAssay Description:Displacement of fluorescent tracer 4-(4-(1-(4-(1-(6-(3-carboxylato-4-(3-iminio-3H-xanthen-9-yl)benzamido)hexyl)-4,5-dihydro-1H-pyrrol-3-yl)butyl)pipe...More data for this Ligand-Target Pair
Target5-hydroxytryptamine receptor 1B(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Displacement of [3H]-5-CT from human 5-HT1B receptor assessed as inhibition constant by radioligand displacement assayMore data for this Ligand-Target Pair
TargetMuscarinic acetylcholine receptor M5(Human)
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
National Institute of Diabetes and Digestive and Kidney Diseases
Curated by ChEMBL
Affinity DataKi: 6.00E+3nMAssay Description:Displacement of [3H]-Ketanserin from human muscarinic M5 receptor assessed as inhibition constant by radioligand displacement assayMore data for this Ligand-Target Pair