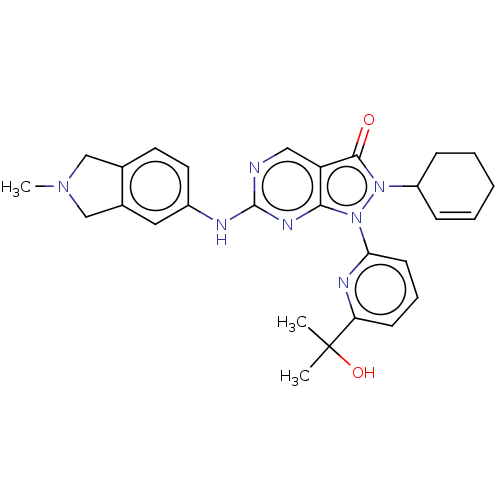
BDBM50615035 CHEMBL5291431
SMILES CN1Cc2ccc(Nc3ncc4c(n3)n(-c3cccc(n3)C(C)(C)O)n(C3CCCC=C3)c4=O)cc2C1
InChI Key InChIKey=OLLXANSJPNHXRT-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50615035
Found 3 hits for monomerid = 50615035
Affinity DataIC50: 2.50E+4nMAssay Description:Inhibition of human N-terminal His6-tagged recombinant full-length PLK1 expressed in baculovirus infected Sf9 cells incubated for 15 mins followed by...More data for this Ligand-Target Pair
Affinity DataIC50: 4.5nMAssay Description:Inhibition of human full length GST-tagged WEE1 expressed in insect cells incubated for 15 mins followed by ATP addition by ADP Glo luminescent assayMore data for this Ligand-Target Pair
Affinity DataIC50: 267nMAssay Description:Inhibition of WEE1 in human U2OS cells incubated for 1 hr by mesoscale discovery assayMore data for this Ligand-Target Pair