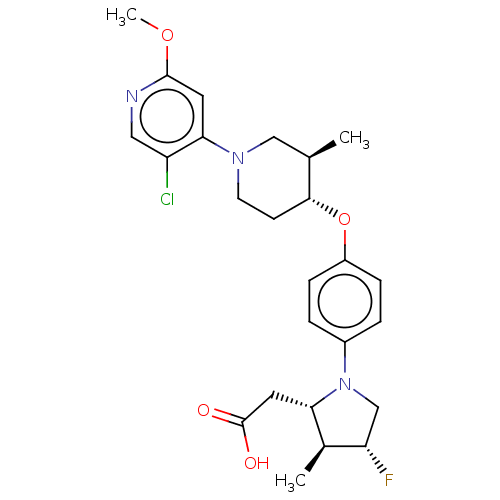
BDBM50614648 CHEMBL5283236
SMILES COc1cc(N2CC[C@@H](Oc3ccc(cc3)N3C[C@H](F)[C@@H](C)[C@@H]3CC(O)=O)[C@H](C)C2)c(Cl)cn1
InChI Key InChIKey=CYIZNYHHAGFMBN-UHFFFAOYSA-N
Data 3 EC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50614648
Found 3 hits for monomerid = 50614648
Affinity DataEC50: >4.80E+4nMAssay Description:Transactivation of PPARgamma (unknown origin)More data for this Ligand-Target Pair
Affinity DataEC50: 29nMAssay Description:Positive allosteric modulation of human GPR40 by calcium based FLIPR analysisMore data for this Ligand-Target Pair
Affinity DataEC50: 48nMAssay Description:Positive allosteric modulation of mouse GPR40More data for this Ligand-Target Pair