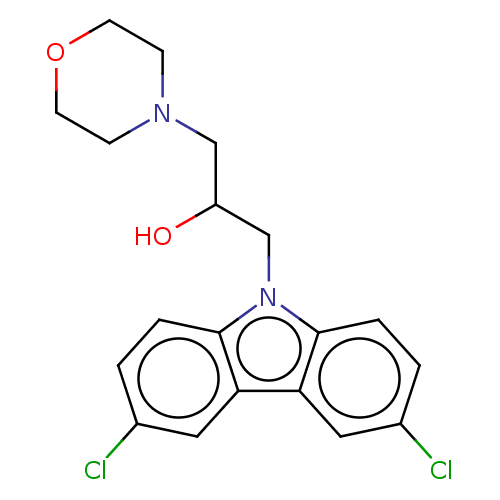
BDBM50613727 CHEMBL4548520
SMILES OC(CN1CCOCC1)Cn1c2ccc(Cl)cc2c2cc(Cl)ccc12
InChI Key InChIKey=MQIMMVBJAICTOC-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50613727
Found 3 hits for monomerid = 50613727
Affinity DataIC50: 6.90E+3nMAssay Description:Inhibition of Saccharomyces cerevisiae H+ ATPase pma1 isoform assessed as ATP hydrolysis incubated for 30 mins by colorimetric methodMore data for this Ligand-Target Pair
Affinity DataIC50: 2.97E+4nMAssay Description:Allosteric inhibition of H+ ATPase pma1 isoform by measuring NADP-coupled ATPase activity in presence of 1 mM ATP by spectrophotometry methodMore data for this Ligand-Target Pair
Affinity DataIC50: 1.68E+4nMAssay Description:Allosteric inhibition of H+ ATPase pma1 isoform by measuring NADP-coupled ATPase activity in presence of 10 mM ATP by spectrophotometry methodMore data for this Ligand-Target Pair