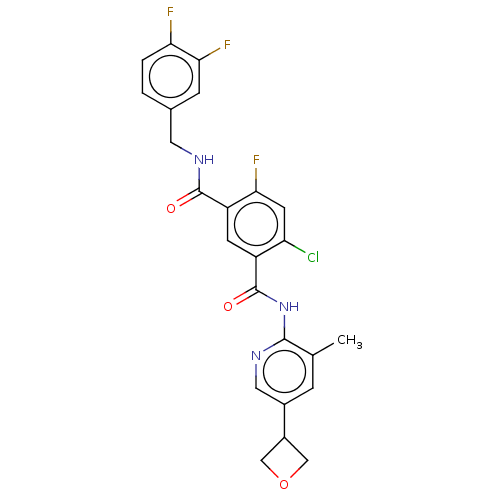
BDBM50610166 CHEMBL5282503
SMILES Cc1cc(cnc1NC(=O)c1cc(C(=O)NCc2ccc(F)c(F)c2)c(F)cc1Cl)C1COC1
InChI Key InChIKey=BUUONEGZVMEGFZ-UHFFFAOYSA-N
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50610166
Found 2 hits for monomerid = 50610166
TargetPeroxisome proliferator-activated receptor gamma(Human)
Institute of Mit and Harvard
Curated by ChEMBL
Institute of Mit and Harvard
Curated by ChEMBL
Affinity DataEC50: 79nMAssay Description:Inverse agonist activity at GST tagged human PPARgamma LBD (231 to 505 residues) expressed in Escherichia coli BL21(DE3) assessed as recruitment of f...More data for this Ligand-Target Pair
TargetPeroxisome proliferator-activated receptor gamma(Human)
Institute of Mit and Harvard
Curated by ChEMBL
Institute of Mit and Harvard
Curated by ChEMBL
Affinity DataIC50: 5nMAssay Description:Inverse agonist activity at human PPARgamma in human RT112/84 transfected with NLuc-fused FABP4 assessed as reduction in PPARgamma transactivation in...More data for this Ligand-Target Pair