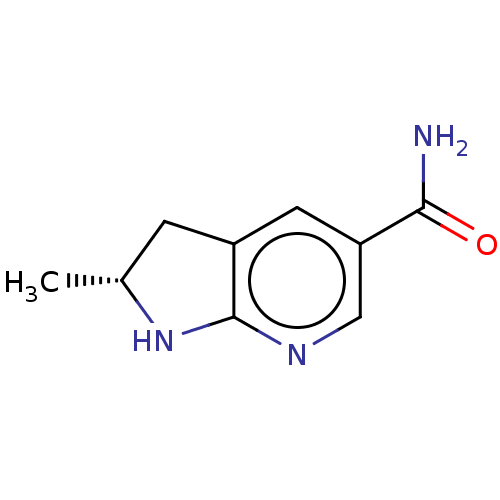
BDBM50607543 CHEMBL5220590
SMILES C[C@@H]1Cc2cc(cnc2N1)C(N)=O
InChI Key InChIKey=HLBSXLCCTVSVBK-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50607543
Found 4 hits for monomerid = 50607543
Affinity DataIC50: 42nMAssay Description:Inhibition of human NNMT measured after 20 mins by luminescence based methyltransferase assayMore data for this Ligand-Target Pair
Affinity DataIC50: 38nMAssay Description:Inhibition of human NNMT in human K562 cells measured after 24 hrs by quantitative mass spectrometryMore data for this Ligand-Target Pair
Affinity DataIC50: 1.00E+5nMAssay Description:Inhibition of CYP450 (unknown origin)More data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
Harvard University
Curated by ChEMBL
Harvard University
Curated by ChEMBL
Affinity DataIC50: 3.00E+4nMAssay Description:Inhibition of hERGMore data for this Ligand-Target Pair