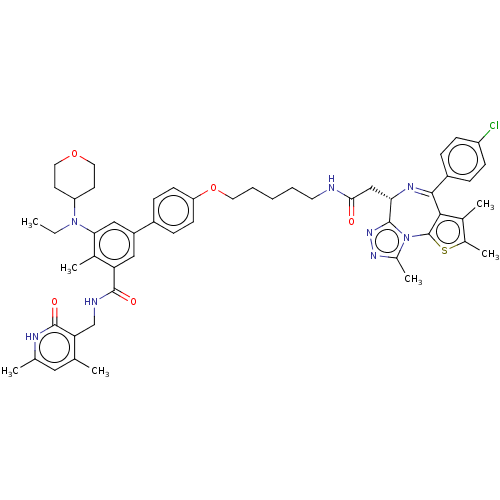
BDBM50605635 CHEMBL5189126
SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(OCCCCCNC(=O)C[C@@H]2N=C(c3c(C)c(C)sc3-n3c(C)nnc23)c2ccc(Cl)cc2)cc1
InChI Key InChIKey=UOTPJXAROHNZGU-UHFFFAOYSA-N
Data 4 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 4 hits for monomerid = 50605635
Found 4 hits for monomerid = 50605635
TargetHistone-lysine N-methyltransferase EZH2(Human)
Sun Yat-Sen University Cancer Center
Curated by ChEMBL
Sun Yat-Sen University Cancer Center
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Inhibition of EZH2 (unknown origin) using H3-derivede peptide (21 to 44) as substrate incubated for 60 min in presence of SAM by MTase-Glo assayMore data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Displacement of fluorescein-labeled JQ1 from His6/TEV cleavage site fused human BRD4 (residues N44 to E168) expressed in Escherichia coli BL21 (DE3) ...More data for this Ligand-Target Pair
Affinity DataIC50: 34nMAssay Description:Inhibition of BRD4 (unknown origin) expressed in Escherichia coli BL21 (DE3) incubated for 30 mins by TR-FRET assayMore data for this Ligand-Target Pair
TargetHistone-lysine N-methyltransferase EZH2(Human)
Sun Yat-Sen University Cancer Center
Curated by ChEMBL
Sun Yat-Sen University Cancer Center
Curated by ChEMBL
Affinity DataIC50: 490nMAssay Description:Inhibition of EZH2 methyltransferase activity in human A2780 cells assessed as reduction in H3K27 level and measured after 96 hrs by Western blot ana...More data for this Ligand-Target Pair