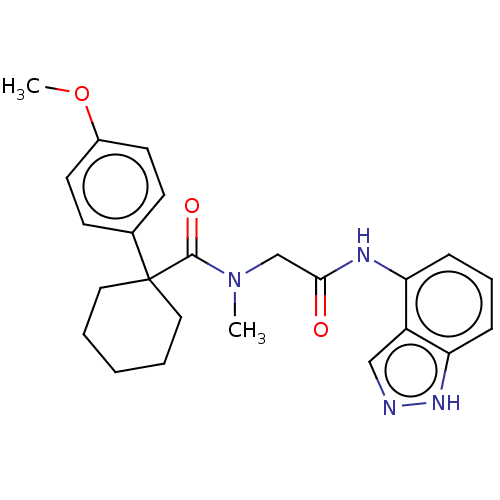
BDBM50600298 CHEMBL5187549
SMILES COc1ccc(cc1)C1(CCCCC1)C(=O)N(C)CC(=O)Nc1cccc2[nH]ncc12
InChI Key InChIKey=QEJJXNXKQNDITO-UHFFFAOYSA-N
Data 2 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 2 hits for monomerid = 50600298
Found 2 hits for monomerid = 50600298
Affinity DataIC50: 190nMAssay Description:Inhibition of N-terminal His tagged human EP300 (1159-1666 residues) expressed in Escherichia coli BL21 (DE3) using biotinylated H4 (1 to 25)-GSGSK p...More data for this Ligand-Target Pair
Affinity DataIC50: 1.01E+3nMAssay Description:Inhibition of EP300 in human LK2 cells assessed as reduction in intracellular histone H3 acetylation at lysine 27 residue incubated for 3 hrs by chem...More data for this Ligand-Target Pair