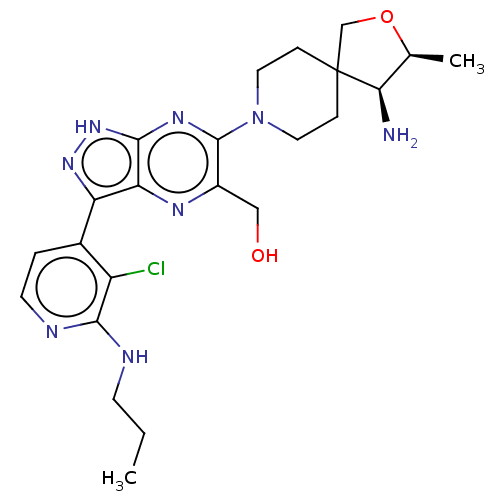
BDBM50588244 CHEMBL5176625
SMILES CCCNc1nccc(-c2n[nH]c3nc(N4CCC5(CO[C@@H](C)[C@H]5N)CC4)c(CO)nc23)c1Cl
InChI Key InChIKey=QGJXABHATSMIPN-UHFFFAOYSA-N
Data 3 IC50
Activity Spreadsheet -- Enzyme Inhibition Constant Data from BindingDB
 Found 3 hits for monomerid = 50588244
Found 3 hits for monomerid = 50588244
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 4nMAssay Description:Inhibition of N-terminal his6-tagged wild type recombinant human SHP2 (1 to 597 residues) expressed in Escherichia coli BL21 (DE3) cells using fluoro...More data for this Ligand-Target Pair
TargetTyrosine-protein phosphatase non-receptor type 11(Human)
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 2nMAssay Description:Inhibition of human SHP2 assessed as downregulation of PERK level in human KYSE520 cells incubated for 2 hrs by Alpha screen assayMore data for this Ligand-Target Pair
TargetPotassium voltage-gated channel subfamily H member 2(Human)
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
University of Texas Md Anderson Cancer Center
Curated by ChEMBL
Affinity DataIC50: 1.60E+3nMAssay Description:Inhibition of hERG ion channel incubated for 3 hrs by fluorescence polarization assayMore data for this Ligand-Target Pair